International researchers have just discovered a new ice form called ice XXI, which compresses water to extreme pressures while keeping it at room temperature.
The achievement was accomplished at the European XFEL, a research facility that houses the world’s most powerful X-ray laser, and DESY’s PETRA III photon source in Hamburg, Germany.
As per the research team, it offers unprecedented insight into how water behaves under conditions similar to those found deep inside icy moons and exoplanets.
“Our findings suggest that a greater number of high-temperature metastable ice phases and their associated transition pathways may exist, potentially offering new insights into the composition of icy moons,” Rachel Husband, PhD, from the DESY HIBEF team, explained.
Discovery under pressure
Warm ice is a high-pressure form of solid water that remains stable at room temperature instead of freezing or melting under normal conditions.
Meanwhile, water, one of Earth’s most familiar substances, comprises two hydrogen (H) atoms and one oxygen (O) atom. It can crystallize into more than 20 known solid phases, each with a unique molecular structure.
However, scientists led by the Korea Research Institute of Standards and Science (KRISS) have now identified a 21st phase of water that challenges existing models of ice formation.
The new form, ice XXI, is structurally distinct from all previously known ice phases. It forms when water is rapidly compressed to supercompressed water at room temperature.
 Researchers led by scientists from the Korea Research Institute of Standards and Science (KRISS) have identified and described a new phase of ice called ice XXI.
Researchers led by scientists from the Korea Research Institute of Standards and Science (KRISS) have identified and described a new phase of ice called ice XXI.
Credit: European XFEL
The new phase is also metastable. This means it can exist for some time even though another form of ice would be more stable at those conditions. The results deepened the researchers’ understanding of ice formation under high pressure.
“Rapid compression of water allows it to remain liquid up to higher pressures, where it should have already crystallized to ice VI,” Geun Woo Lee, PhD, one of the scientists at KRISS, elaborated.
According to Lee, ice VI is particularly fascinating. Scientists believe it exists deep within icy moons like Titan and Ganymede. Its highly distorted structure could enable complex transition pathways that lead to metastable ice phases.
Unveiling ice XXI
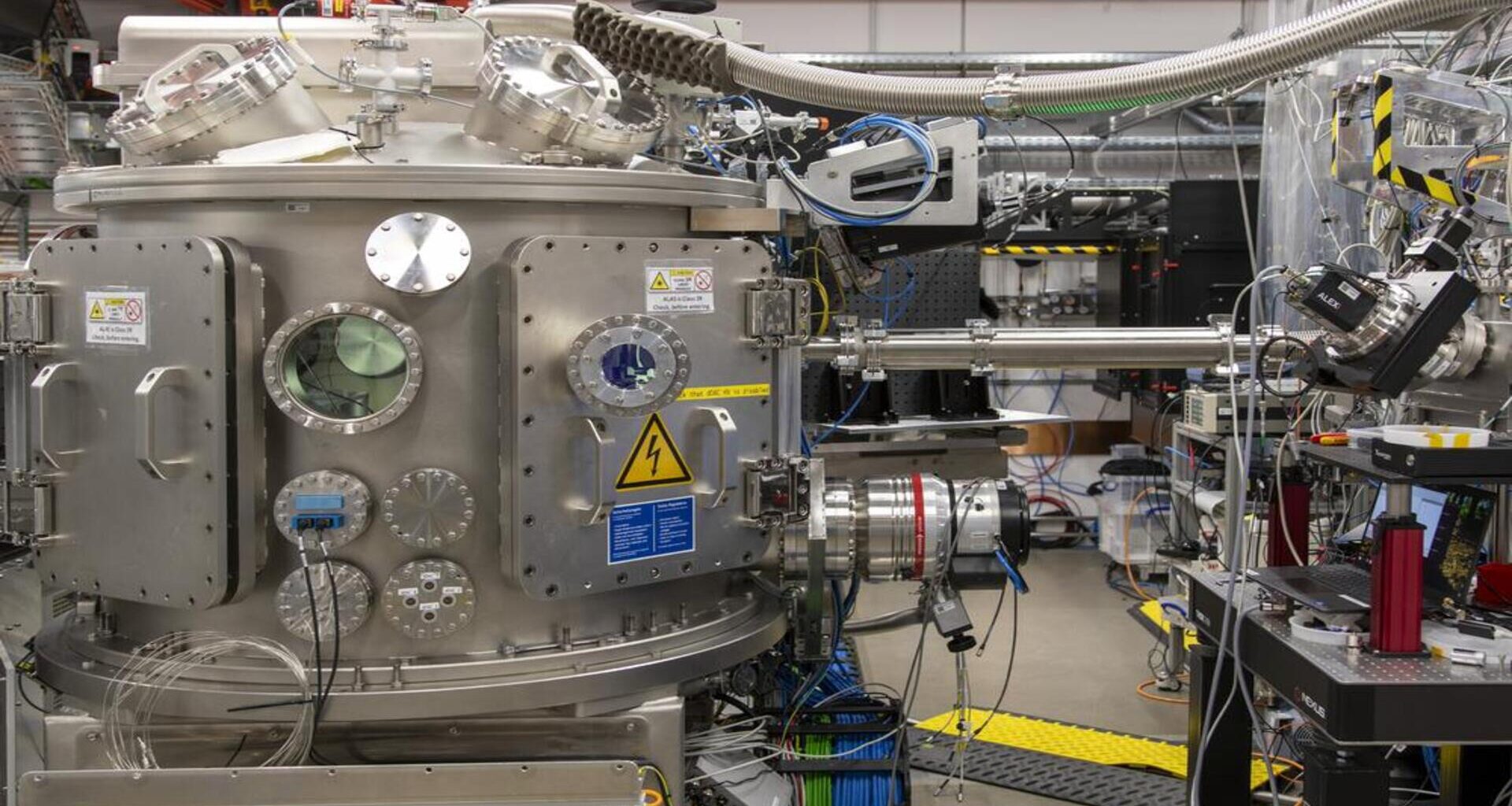
For the research, the team used a diamond anvil cell, a device that can generate enormous pressures by squeezing a sample between two opposing diamonds, to recreate extreme planetary conditions.
Inside this cell, they compressed water up to two gigapascals (GPa), about 20,000 times more than normal air pressure. Under these pressures, water normally turns into ice VI. However, when compressed extremely quickly, within just 10 milliseconds, the liquid remained stable long enough to transition into the newly observed ice XXI.
To capture the moment of crystallization, the team then turned to the European XFEL, whose ultrashort X-ray pulses can record atomic-scale events millions of times per second.
Like a high-speed camera, the XFEL enabled the scientists to film the rapid transformations as water molecules locked into their new arrangement.
Follow-up experiments at the P02.2 beamline at PETRA III confirmed that ice XXI has a tetragonal crystal structure, built from surprisingly large repeating units known as unit cells.
“With the unique X-ray pulses of the European XFEL, we have uncovered multiple crystallization pathways in H2O, which was rapidly compressed and decompressed over 1000 times using a dynamic diamond anvil cell,” Lee said. “The structure in which liquid H2O crystallizes depends on the degree of supercompression of the liquid.”
Meanwhile, Cornelius Strohm, PhD, from the DESY HIBEF team, said samples are placed between the tips of two opposing diamond anvils in this specialized pressure cell. This allows them to be compressed precisely along a controlled pressure pathway.
“It is fantastic to see another great outcome from our Water Call, an initiative inviting scientists to propose innovative studies on water,” Husband stated in a press release. “We are looking forward to many more exciting discoveries ahead.”
The study has been published in the journal Nature Materials.