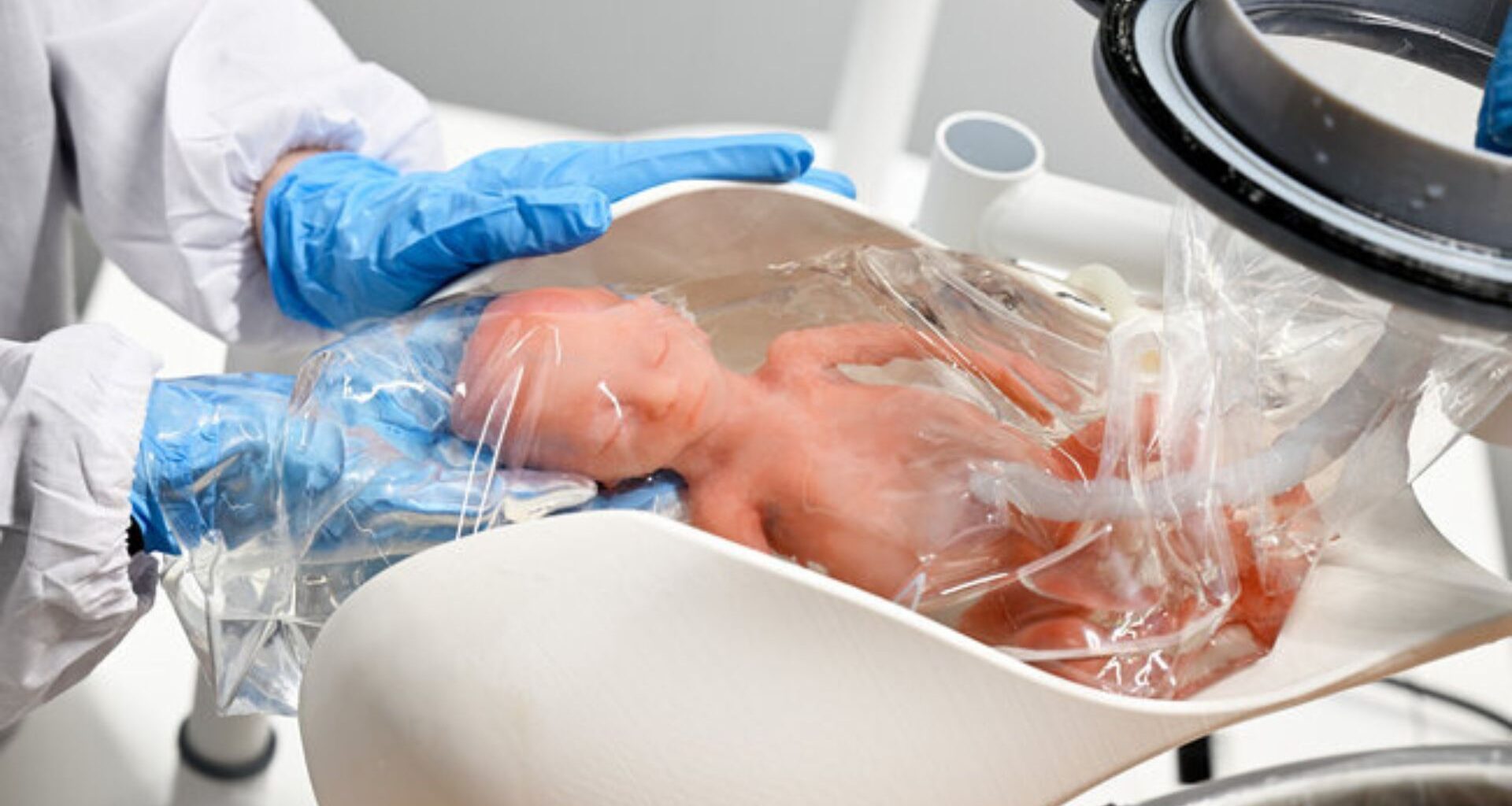
A Netherlands-based startup, Aqua Womb, is exploring the potential of making a womb-like life support system for extremely premature infants.
The objective is to advance the care of premature newborns through the development of a clinical-grade artificial womb, also known as a liquid-filled incubator.
Babies born between 22 and 24 weeks face huge risks. They currently have a low chance of survival and a high likelihood of developing health issues like chronic lung disease and neurological damage.
In the US, thousands of infants are born each year within the 22-24 week window. Premature birth is the nation’s second-leading cause of infant death.
One major reason why preterm infants are unable to sustain life is that their lungs are too immature to breathe air.
Current mechanical ventilators can risk injuring fragile, underdeveloped lungs.
The artificial system is designed to provide a safe, continuous shelter environment, protecting the infant from early exposure to air.
It could allow the baby to float, grow, and mature without ever coming into contact with air.
A new womb outside the mother’s body
Interestingly, this new device could allow premature babies to continue to grow immersed in an artificial womb environment until their lungs are mature enough to function.
The Guardian reported that the system utilizes a glass tank filled with synthetic amniotic fluid at near-body temperature.
It is designed with a firm outer silicone layer that pushes back against the baby’s kicks, thereby helping their muscles to stretch and gain strength.
An artificial womb would allow the baby to be delivered via C-section directly into a fluid-filled pouch and connected to a human-made placenta.
Clinicians would then quickly connect the umbilical cord to the external life support system or artificial placenta.
The artificial placenta, roughly the size of a human fist, serves as the central hub of life support, mimicking the exchange functions of a biological placenta.
It is engineered with two types of specialized tubing: extremely fine catheters are used to gently extract carbon dioxide and metabolic waste from the infant’s bloodstream, while more robust cannulas simultaneously infuse the blood with the necessary oxygen and nutrients.
This complex, high-stakes reconnection ensures continuous circulation and sustainment, providing the premature infant the precious time needed for internal organs to mature without the damaging stress of air breathing.
Clinical trials approval
For parents who have suffered the devastation of extreme prematurity, the technology represents a long-awaited miracle to give infants more time in the womb.
If successful, the technology could save more vulnerable babies and spare them from chronic complications.
One mother, who lost a 22-week-old child, said she would have “signed anything” to access the device.
However, the technology is not arriving anytime soon, and it comes with deep questions. U.S. health regulators are actively involved.
According to reports, the U.S. Food and Drug Administration (FDA) is actively reviewing the data and considering whether to approve the first clinical trials.
In September 2023, the FDA convened an advisory committee to consider the trials.
Beyond the technical hurdles, experts warn that the artificial womb creates a new ethical frontier.
“This kind of device would create a new stage of human development, something we’ve never had to describe or regulate before,” Elizabeth Chloe Romanis, a medical law scholar at Durham University, told the Guardian.
The technology is not ready yet, but the world waits for this potential miracle.
AquaWomb has not yet disclosed when the human trials are expected to begin.