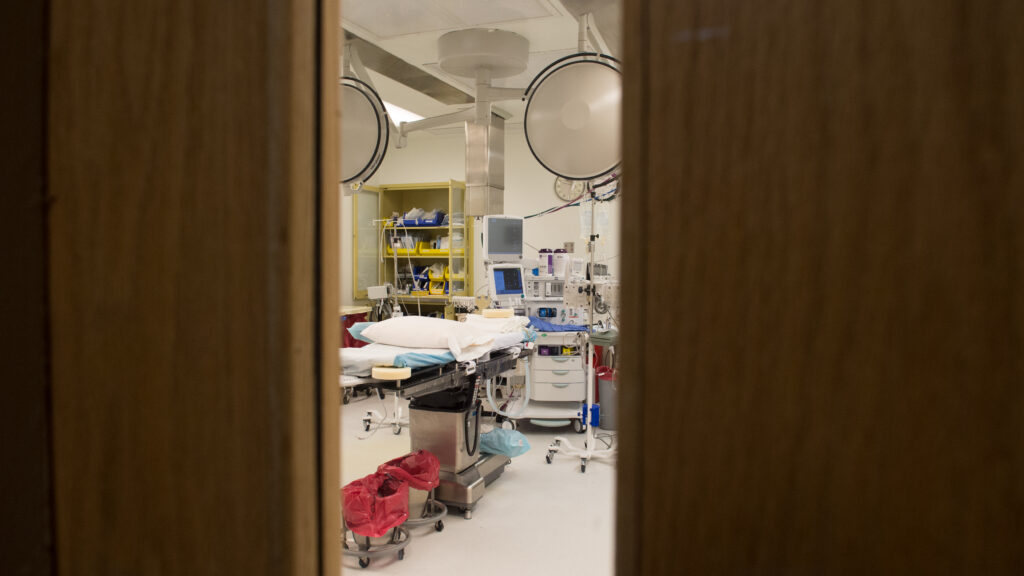
The Food and Drug Administration’s breakthrough device program continues to expand at a breakneck pace. Established to grease the wheels of regulatory conversation and submission for innovative devices that promise to help patients with debilitating disease, the FDA has stamped 1,176 products with the breakthrough label — and so far authorized 160 of them to enter the market.
As those 160 devices enter doctors’ offices and surgical suites, their implications for health care costs are expanding. In bipartisan bills before Congress, breakthrough devices are proposed to receive four years of automatic Medicare coverage. It’s an idea that has come and gone since the previous Trump administration, thanks in part to objections that a “breakthrough” label doesn’t guarantee better outcomes for patients than existing options. Now, health policy researchers are raising the alarm again.
With the regulatory flexibilities the FDA leverages when authorizing breakthrough devices, “we’re concerned that this will ultimately lead to low value or potentially harmful spending and utilization of devices that come to market with less rigorous evidence to support their use,” said physician Vinay Rathi, a health policy researcher at Ohio State University.
STAT+ Exclusive Story
Already have an account? Log in
This article is exclusive to STAT+ subscribers
Unlock this article — and get additional analysis of the technologies disrupting health care — by subscribing to STAT+.
Already have an account? Log in
Individual plans
Group plans
To read the rest of this story subscribe to STAT+.