Clinical Trial Materials Manufacturing Market Size and Forecast 2025 to 2034
The global clinical trial materials manufacturing market is expanding with growing demand for efficient supply chains and customized solutions in clinical research. The clinical trial material manufacturing market is expanding due to increased R&D investments, rising chronic disease prevalence, and the globalization of clinical trials, driving demand for efficient, compliant, and scalable supply solutions.
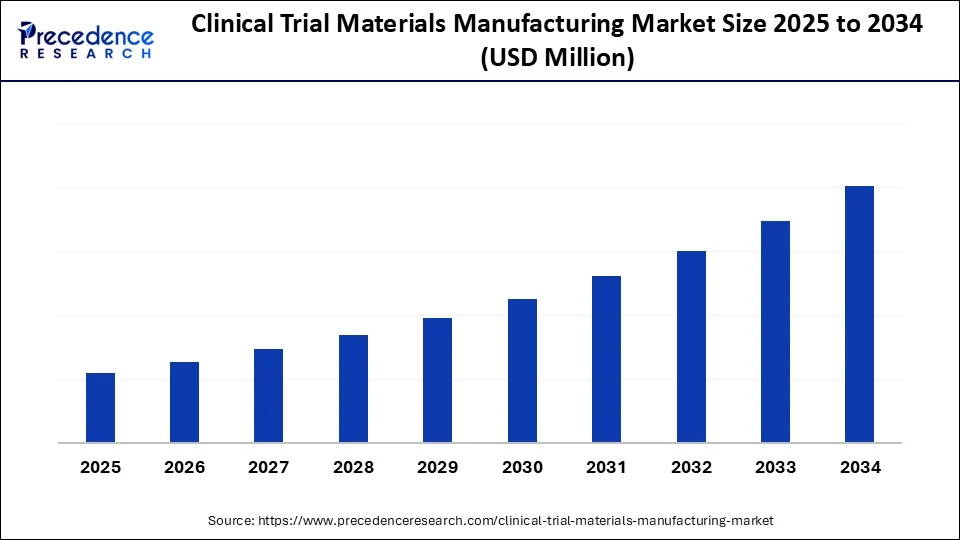
Clinical Trial Materials Manufacturing Market Key Takeaways
North America dominated the clinical trial materials manufacturing market with the largest market share of 35% in 2024.
Asia Pacific is estimated to expand the fastest CAGR in the market between 2025 and 2034.
By product type, the investigational medicinal products segment held the biggest market share of 70% in 2024 the market share and is also anticipated to grow at a remarkable CAGR between 2025 and 2034.
By service type, the manufacturing segment captured the highest market share of 40% in 2024.
By service type, the packaging segment is expected to expand at a notable CAGR over the projected period.
By clinical trial phase, the phase III segment contributed the major market share of 52.7% in 2024.
By clinical trial phase, the phase I segment is expected to expand at a notable CAGR over the projected period.
By therapeutic area, the oncology segment held the maximum market share of 38.8% in 2024.
By therapeutic area, the cardiovascular segment is expected to expand at a notable CAGR over the projected period.
By end-user, the pharmaceutical companies segment generated the major market share of 50% in 2024.
By end user, the biotechnology firm segment is expected to expand at a notable CAGR over the projected period.
How is Artificial Intelligence Impacting Clinical Trial Material Manufacturing?
Artificial intelligence is changing the way clinical trial materials are manufactured by improving precision in drug supply, forecasting, and material handling. Platforms like Octozi automate data cleaning, which significantly reduces manual errors in manufacturing records.
Similarly, in July 2025, QuantHealth, the AI-powered clinical simulation company, announced it had successfully simulated the results from over 350 clinical trials, achieving up to 90 percent predictive accuracy. This helps manufacturers predict material demand and cut down on costly overproduction. (Source: https://www.businesswire.com)
On the supply side, pharmaceutical companies are using AI-driven systems for real-time demand forecasting. This ensures that investigational medicinal products are delivered on time and reduces waste. These advancements show how AI is playing a bigger role in improving efficiency, sustainability, and regulatory compliance in the clinical trial materials manufacturing market. (Source: https://arxiv.org)
Market Overview
Clinical trial materials manufacturing market materials manufacturing refers to the specialized process of producing, packaging, labeling, and distributing all materials required to conduct clinical trials. This includes investigational medicinal products (IMPs), placebos, comparator drugs, and ancillary materials (such as laboratory kits, reagents, and medical devices) that are used in the testing of new drugs or therapies. The manufacturing process ensures that all materials meet stringent regulatory standards (GMP, GCP, GDP) for quality, safety, and efficacy, while also adhering to specific temperature, packaging, and labeling requirements to maintain stability and integrity throughout the trial lifecycle.
Clinical Trial Materials Manufacturing Market Growth Factors
Increasing Global Clinical Trials- The increase in the volume of clinical trials, with escalating chronic disease and global drug development, ultimately creates a greater need for effective CTMS solutions to manage the complexities of the trial operations.
Advancements in Manufacturing Technology: Automation, serialization, and cold chain logistics improve production efficiency, compliance, and safety of trial material.
Regulatory Compliance Pressure- Increased pressure to adhere to strict regulatory compliance frameworks, like FDA 21 CFR Part 11 and GDPR, increases the preferred use of material that helps ensure compliant, standardized, and auditable documentation in clinical trials.
Growing Outsourcing to Contract Research Organizations- Many pharmaceutical companies are outsourcing their trials to Contract Research Organizations (CROs), for specialized clinical trial material to manage a single source of truth for trial project tracking, budgets, and communication.
Market Scope
Report Coverage
Details
Dominating Region
North America
Fastest Growing Region
Asia Pacific
Base Year
2024
Forecast Period
2025 to 2034
Segments Covered
Product Type, Service Type, Clinical Trial Phases, Application,Therapeutic Area, End User and Region
Regions Covered
North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa
Market Dynamics
Drivers
How is Outsourcing Creating Growth in Clinical Trial Material Manufacturing?
The primary growth driver in the clinical trial materials manufacturing market is the growing outsourcing of drug development and related services to specialized manufacturing organizations. As clinical trials become increasingly elaborate and global, pharmaceutical companies are finding themselves challenged to achieve consistent quality, regulatory compliance, and well-timed supply of investigational materials. To address these challenges, pharmaceutical companies are increasingly partnering with Contract Development and Manufacturing Organizations (CDMOs) and Contract Manufacturing Organizations (CMOs). The rise in outsourced clinical trial material manufacturing is notable and reflected in several recent developments described below.
Catalent was acquired by Novo Holdings in March 2024 for US$16 billion, reflecting the continued demand for outsourcing the expertise in these areas. In many cases, reliance on external partners provides flexibility, cost savings, and timelines that benefit different parties involved in clinical development and trial execution, making outsourcing an extremely powerful growth driver as a market category. (Source: https://www.contractpharma.com)
Restraint
How are Supply Chains for Clinical Trial Materials Being Threatened by Geopolitical Unrest?
The production and distribution of clinical trial materials are being increasingly disrupted by geopolitical tensions. For example, growing trade tensions between the United States and China have caused pharmaceutical companies to reevaluate their dependence on Chinese suppliers. Some companies are looking into local testing options to prevent delays and cost increases, while others, like WuXi Biologics, are modifying project plans and storing materials to mitigate potential disruptions.
Trial budgets and schedules may be impacted by shortages of essential materials brought on by these geopolitical uncertainties. For instance, in 2024, there were more than 300 drug shortages in the US, which was a record high. These shortages included necessary prescription drugs for diseases like diabetes, cancer, and asthma. These interruptions highlight the necessity for pharmaceutical firms to diversify their supply chains and create plans. (Source: https://hbr.org)
Opportunity
Could Flexible, On-Demand Manufacturing Be the Game-Changer for Clinical Trial Materials?
Ever since trial needs evolved, the power of flexible, on-demand manufacturing has emerged as a huge opportunity in the clinical trial material (CTM) field. This approach produces CTMs in smaller, agile batches instead of large runs. It boosts trial efficiency and reduces waste. It also allows for quick adjustments to formulation or dosing changes during the trial. Recently, global pharma leaders have started responding. Takeda is looking into India’s cost-effective and varied trial ecosystem. The company is expanding R&D hubs and accelerating the rollout of global drugs, including oncology and dengue vaccines.
Product Type Insights
Why In Investigational Medicinal Products Have the Largest Share of Product Type?
The investigational medicinal products (IMPs) segment is important for clinical studies because it facilitates the testing of new drugs and therapies for regulatory approval. With the number of drug development pipelines continuing to grow, the increased prevalence of chronic conditions, and increased investment in novel therapeutics, the demand for IMPs will remain strong and will continue to hold the lead.
Service Type Insights
Why is Manufacturing the Leading Service Type in the Clinical Trial Materials Manufacturing Market?
The manufacturing segment continued to dominate the clinical trial materials manufacturing market in 2024. This growth is attributed to the increasing number of clinical trials being conducted, and the corresponding need for large-scale production of trial materials, and guarantees of quality and compliance. Pharmaceutical and biotech companies are turning to specialized manufacturing services to meet the regulatory requirements, in a timely manner, of investigational products, creating a firm foundation for the segment.
The packaging segment is expected to be the fastest-growing segment. The emergence of decentralized trials, patient-centric study models, and global networks has created a strong need for reliable, innovative, and flexible packaging solutions. Packaging features such as tamper-evidence, temperature control, and patient-centric solutions will be increasingly necessary, creating rapid growth, and packaging will be considered a major service in clinical trial material manufacturing.
Clinical Trial Phases Insights
What Makes Phase III Dominant Clinical Trial Phases in the Clinical Trial Materials Manufacturing Market?
The Phase III segment accounts for the largest share of the clinical trial materials manufacturing market in 2024. Phase III trials are large studies that require the highest volume of clinical trial materials, as they typically involve studies on large subject populations across multiple investigator sites. Given their high costs, high regulatory scrutiny, and large-scale production of investigational product, Phase III trials are the most resource-intensive phase of the clinical trial process, which is why they dominate the clinical trial materials manufacturing.
In contrast, the Phase I segment is the fastest-growing category. Due to the increasing importance of precision medicine, biologics, and advanced therapies, early-stage studies are on the critical path for safety and dosage assessments. Increased investments in innovative drug candidates and accelerated trial approvals are helping to drive growth for Phase I trials, and this area will remain the key growth driver in the coming years.
Therapeutic Area Insights
What Makes Oncology the Most Dominant Therapeutic Area in the Clinical Trial Materials Manufacturing Market?
The oncology segment dominated the clinical trial materials manufacturing market in 2024, and is the largest domain. The growing cancer burden and continual advancements of new targeted therapies and immunotherapies have driven an increased number of oncology trials. Due to the inherent complexity of cancer treatments and urgent demand for oncology solutions, oncology is in the highest demand for clinical trial material and thus continues to lead the market.
The cardiovascular segment is expected to evolve the fastest. The growing prevalence of heart disorders and the expansion of innovative therapies, including gene therapies and advanced biologics, are driving growth in cardiovascular. The emphasis on expanding clinical research in cardiovascular health and high mortality rates has cultivated one of the fastest-embracing domains.
End User Insights
Which End-User Dominated the Clinical Trial Materials Manufacturing Market in 2024?
The pharmaceutical companies segment dominated the end-user segment in 2024. The end-user segment of clinical trial material manufacturing is primarily composed of pharmaceutical companies. A heavy investment in R&D and the presence of large pipelines of new drugs require the production of investigational materials in a compliant and timely manner. Pharmaceutical companies often can rely on their existing global network and have more regulatory knowledge than other industries. As a group, pharmaceutical companies are the largest category of end-users of clinical trial manufacturing services.
The biotechnology firm segment is growing at a faster rate. The growth in the number of start-ups and small to mid-sized biotech firms developing biologics, cell therapies, and gene therapies supports the need for specialized clinical trial materials. While their limited internal manufacturing capacity may be seen as a hindrance, reliance on outsourcing partners is contributing to their embrace of advanced manufacturing services, which suggests that biotechnology firms are one of the fastest-growing end-user segments.
Regional Insights
Why is North America at the Forefront of the Clinical Trial Materials Manufacturing Market?
North America’s dominance comes from a strong network of large biopharma companies, established CDMOs and CROs, efficient cold-chain logistics, and close ties with academic research centers and regulators. The U.S. market benefits from high research and development budgets, many early-phase studies that need complex investigational medicinal product (IMP) manufacturing, including biologics and cell therapies, and increased efforts to bring production back to the U.S. after COVID-related supply-chain issues. Interest from private equity and strategic mergers and acquisitions is consolidating capacity, boosting manufacturing scale and skills, which keeps clinical-supply production focused in the region.
Why is the U.S. leading in the North American clinical trial materials manufacturing market?
The U.S. leads because it has the highest number of sponsor companies, specialized CDMOs for biologics and aseptic fill and finish, and clinical supply logistics providers. A strong regulatory framework, extensive investigator networks, and the highest per-capita spending on R&D speed up the start of protocols and increase the demand for complex IMP batches. The large registry on www.ClinicalTrials.gov and ongoing platform improvements show the volume of U.S. trials and the operational expertise needed to support them. Recent industry insights also highlight that strategic investments in domestic capacity and automation are key to maintaining leadership.
Why is Asia Pacific the Fastest-Growing Region for Clinical Trial Material Manufacturing?
Asia Pacific is becoming the fastest-growing center due to its large patient population, cost-effective trial operations, and significant growth of CDMO and CDPO infrastructure. Multinational sponsors are increasingly moving parts of their supply chains to this region to cut development costs and speed up timelines, especially for biologics and oncology trials. Governments across APAC are simplifying regulatory frameworks and investing in clinical supply logistics. The region also benefits from increasing biotech innovation and stronger local manufacturing systems, making it a preferred choice for future trial material production.
Why is China the leading country in Asia Pacific?
China stands out in Asia by combining the world’s fastest-growing clinical trial environment with major investments in biologics manufacturing capacity. Recent data shows that China has surpassed the U.S. in the number of registered clinical trials, highlighting its aggressive growth strategy. Domestic CDMOs are expanding their aseptic fill-finish and biologics capabilities to support both local and global sponsors. Government-backed initiatives are cutting approval timelines and improving GMP compliance. With growing venture capital investments and strong biotech pipelines, China has become the main hub driving Asia Pacific’s leadership in clinical trial material manufacturing.
Value Chain Analysis
Sourcing of Raw Materials & Supplier Qualification
This phase entails qualifying and sourcing suppliers of active pharmaceutical ingredients (APIs), excipients, and packaging materials that adhere to strict GMP requirements. Firms conduct supplier audits, negotiate agreements, and implement contingency sourcing to provide consistent quality, traceability, and a constant supply, which is vital in maintaining rigid clinical trial schedules.
Manufacturing & Formulation Development
During this phase, CTM producers formulate and produce drug products, which are both sterile and non-sterile. The operation includes technology transfer, process optimisation, batch manufacture, aseptic filling, and lyophilisation to have uniformity, stability, and protocol specification compliance to ensure efficacy and safety of the patient.
Quality Control, Stability Testing & Regulatory Compliance
Quality assurance ensures that all clinical trial materials are of high quality, meeting strict analytical and regulatory requirements. This encompasses method validation, batch release testing, stability testing, and extensive documentation. Companies are also responsible for regulatory submissions, inspection readiness, and corrective action, assuring compliance at global trial sites.
Clinical Supply Chain, Packaging & Site Services
This phase is all about the management and distribution of clinical trial materials. Functions comprise cold-chain shipping, kit assembling, labeling, placebo management, inventory management, and resupply on time to sites. Proper management guarantees chain-of-custody integrity, storage, and on-time delivery even in cases of intricate multi-region or adaptive clinical trials.
Clinical Trial Materials Manufacturing Market Companies

Thermo Fisher Scientific,
Catalent,
Parexel International,
Almac Group,
Piramal Group,
Marken (DHL Supply Chain),
PCI Pharma Services,
World Courier,
Recipharm,
IQVIA,
Sharp Clinical,
Syneos Health,
ICON plc,
Biocair,
Yourway,
Patheon (a part of Thermo Fisher),
UDG Healthcare (Ashfield),
Myonex,
Rubicon Research, and
Clinigen
Segments Covered in the Report
By Product Type
Investigational Medicinal Products (IMPs)
Small Molecules
Biologics
Vaccines
Placebos
Comparator Drugs
Ancillary Materials
Medical Devices
Laboratory Kits
Reagents
By Service Type
Manufacturing
Active Pharmaceutical Ingredients (API)
Finished Dosage Forms
Biologics Manufacturing
Packaging
Primary Packaging
Secondary Packaging
Blister Packaging
Bottle Packaging
Sachet Packaging
Labeling
Serialization
Tamper-Evident Labels
Multi-Language Labels
Distribution
Cold Chain Logistics
Ambient Logistics
Just-in-Time Delivery
Global Distribution
Regional Distribution
By Clinical Trial Phase
Phase I
Phase II
Phase III
Phase I
By Therapeutic Area
Oncology
Cardiovascular
Neurology
Infectious Diseases
Rare Diseases
Endocrinology
Immunology
Respiratory
Dermatology
Gastroenterology
By End-User
Pharmaceutical Companies
Biotechnology Firms
Contract Research Organizations (CROs)
Academic Research Institutions
Government Research Bodies
By Region
North America
Europe
Asia-Pacific
Latin America
Middle East and Africa
