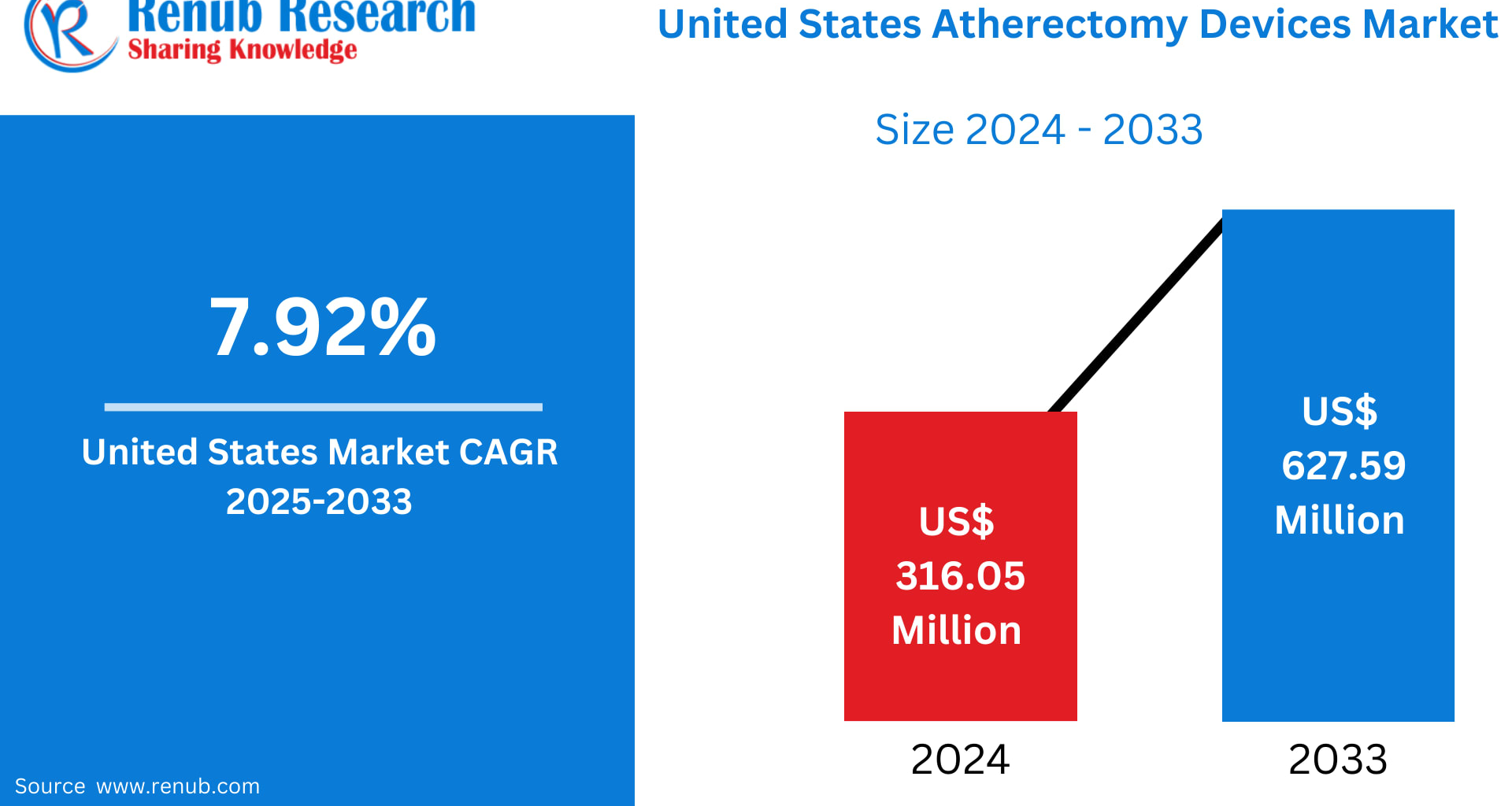
The United States continues to lead the global medical device landscape, especially across cardiovascular technologies where innovation often meets urgent clinical need. The United States Atherectomy Devices Market, driven by a combination of rising disease prevalence, modern procedural approaches, and continued investment in minimally invasive care, is on track for significant expansion.
According to Renub Research, the market is projected to rise from US$ 316.05 Million in 2024 to US$ 627.59 Million by 2033, reflecting a strong CAGR of 7.92% during 2025–2033. This trajectory underscores how atherectomy devices—once niche tools used sparingly—have become crucial components in the treatment of peripheral and coronary artery disease.
From device advancements to state-by-state growth patterns, here’s a comprehensive outlook on what’s driving the U.S. atherectomy boom.
Understanding Atherectomy Devices: A Lifeline for Plaque-Blocked Arteries
Atherectomy devices are specialized systems designed to remove or debulk plaque from the arteries in patients with atherosclerosis. Instead of compressing plaque (as with angioplasty), atherectomy physically removes it, often improving long-term vessel patency and patient outcomes.
In U.S. clinical practice today, atherectomy plays a growing role in:
Percutaneous coronary interventions (PCI)
Peripheral artery interventions (PAI)
Treatment of calcified, fibrotic, or eccentric lesions
Management of in-stent restenosis
Several major atherectomy technologies dominate the market:
Directional atherectomy – shaves and removes targeted plaque
Rotational atherectomy – uses a high-speed burr to break blockages
Orbital atherectomy – utilizes a spinning crown to sand plaque
Laser atherectomy – vaporizes plaque using ultraviolet light
Each device category brings unique strengths, allowing cardiologists and vascular surgeons to tailor interventions based on lesion type and patient anatomy.
Market Growth Drivers: Why Demand is Accelerating
1. Increasing Burden of Peripheral Artery Disease (PAD) and Cardiovascular Illness
More than 12 million Americans suffer from PAD, with many cases going undiagnosed until they progress to advanced stages.
The rise in:
Obesity
Diabetes
Hypertension
Aging populations
Sedentary lifestyles
has created a perfect storm for cardiovascular disease in the United States.
As plaque accumulation becomes more common, so does the need for effective, minimally invasive treatments. Atherectomy devices offer shorter recovery times, reduced hospital stays, and improved outcomes—benefits that resonate strongly with both patients and physicians.
The CDC notes a particularly troubling rise in late-stage PAD, making atherectomy a preferred method for restoring blood flow in complex lesions.
2. Rapid Technological Advancements and Product Innovation
The U.S. is a hub for medical device R&D, and atherectomy technologies have benefitted immensely from this innovation ecosystem.
Modern systems now offer:
Real-time imaging capabilities
Greater crossability and navigation
Reduced procedural complications
Improved plaque removal efficiency
Compatibility with advanced stents and balloons
Laser and orbital atherectomy systems, in particular, have experienced notable upgrades in speed, precision, and safety.
A key milestone arrived in November 2024, when Royal Philips announced the enrollment of the first U.S. patient in its THOR IDE trial. The trial evaluates a next-generation device that combines laser atherectomy with intravascular lithotripsy—a promising hybrid approach for managing severe PAD.
These innovations are driving rapid adoption across U.S. hospitals, outpatient centers, and specialty clinics.
3. Expanding Reimbursement Coverage Across the Healthcare System
Favorable reimbursement is one of the strongest catalysts for growth.
Medicare and private insurers are:
Increasing coverage for atherectomy procedures
Enhancing payment models for outpatient vascular care
Reducing barriers that historically limited patient access
Hospitals and clinics are far more willing to invest in expensive atherectomy platforms when they can be reimbursed reliably. This is especially impactful in the management of older patients, most of whom rely on Medicare and represent the largest share of PAD cases.
Market Challenges: Obstacles Slowing Wider Adoption
1. High Cost of Devices and Procedures
Atherectomy systems are among the most expensive tools used in endovascular care.
Challenges include:
High upfront capital investment for hospitals
Costly disposable components
Patient out-of-pocket expenses despite insurance
Budget constraints in smaller facilities and rural hospitals
These cost pressures threaten equitable access and may temper market penetration in lower-income regions.
2. Procedural Risks and Shortage of Skilled Specialists
Although atherectomy is minimally invasive, it is not risk-free. Potential complications include:
Distal embolization
Arterial perforation
Dissection
Restenosis
Such risks require operators with advanced training—yet the U.S. faces a shortage of interventional cardiologists and vascular surgeons skilled in complex atherectomy.
Rural states and underserved communities feel this gap most acutely. As training programs expand, the market will likely see broader adoption, but progress will remain gradual.
Segment Outlook: Product Types, Applications & End Users
Product Type
Directional
Rotational
Orbital
Laser
Application Areas
Cardiovascular
Neurovascular
Peripheral Vascular
End Users
Hospitals
Ambulatory Care Services
Medical Research Institutes
Directional Atherectomy Devices: Precision Driving Adoption
Directional atherectomy is popular among U.S. physicians due to its unmatched precision. It enables clinicians to remove targeted plaque while preserving healthy vessel tissue—critical in treating complex or eccentric lesions.
Enhanced catheter designs, improved cutter mechanisms, and better integration with intravascular imaging have made directional systems a go-to choice in PAD care. Favorable reimbursement policies further support adoption.
Laser Atherectomy Devices: The Fastest-Growing Segment
Laser atherectomy is gaining significant momentum, particularly for treating:
In-stent restenosis
Long or complex lesions
Heavily calcified blockages
The ability to vaporize rather than mechanically remove plaque reduces trauma to vessel walls. With hospitals and ambulatory centers increasingly equipped for laser procedures—and with ongoing R&D—this segment is expected to claim a rising share of the U.S. market through 2033.
Cardiovascular Atherectomy: Addressing America’s Leading Cause of Death
Cardiovascular diseases remain the number-one cause of death in the United States, making interventions like atherectomy critical tools in cardiology.
Atherectomy is increasingly being used alongside:
Balloon angioplasty
Stent placement
Intravascular imaging
Drug-coated balloons
The aging U.S. population and widespread screening support continuous growth. As clinical evidence backing atherectomy’s efficacy builds, physician confidence and utilization rates continue to rise.
Hospitals: The Backbone of the U.S. Atherectomy Devices Market
Hospitals dominate atherectomy procedure volumes due to their:
Broader device portfolios
Experienced specialists
Stronger financial infrastructure
Access to advanced imaging
Participation in clinical research and trials
Robust Medicare and private insurance support
Hospitals provide an ecosystem where complex cardiovascular cases can be treated safely. They remain, and will continue to be, the largest end-user segment in the market.
State-Level Analysis: California, New York, and New Jersey Lead Growth
California
California’s massive healthcare ecosystem, aging population, and concentration of medical device companies make it a powerhouse market. High rates of diabetes, advanced hospitals, and medical tourism further strengthen demand.
New York
With one of the highest population densities and cardiovascular disease rates, New York depends heavily on atherectomy technologies. Its major hospitals serve as referral hubs for the most complex PAD cases.
New Jersey
Though smaller, New Jersey is a rapidly growing market supported by:
High prevalence of lifestyle-related cardiovascular conditions
Proximity to top-tier medical centers in New York and Philadelphia
Strong insurance networks
Expanding outpatient vascular programs
These states—alongside Texas, Florida, Pennsylvania, Ohio, North Carolina, and others—represent high-value pockets of opportunity for device makers.
Top U.S. States in the Market
The leading states include:
California • Texas • New York • Florida • Illinois • Pennsylvania • Ohio • Georgia • New Jersey • Washington • North Carolina • Massachusetts • Virginia • Michigan • Maryland • Colorado • Tennessee • Indiana • Arizona • Minnesota • Wisconsin • Missouri • Connecticut • South Carolina • Oregon • Louisiana • Alabama • Kentucky • Rest of the U.S.
Key Companies in the U.S. Atherectomy Devices Market
Each profiled across 5 critical viewpoints—Overview, Key Personnel, Recent Developments, SWOT, and Revenue Analysis:
Boston Scientific Corporation
Abbott Laboratories
Medtronic plc
Cardinal Health
Terumo Corporation
Integer Holdings Corporation
Becton, Dickinson and Company (BD)
AngioDynamics Inc.
These companies are continually expanding R&D pipelines, launching next-generation products, and enhancing their market presence through acquisitions and clinical trial leadership.
Final Thoughts: A Market Poised for Robust Long-Term Growth
The United States Atherectomy Devices Market is entering a transformative decade. With cardiovascular disease rates rising and the demand for minimally invasive interventions increasing, the industry is primed for sustained expansion through 2033.
Key strengths—such as strong reimbursement frameworks, active R&D pipelines, and advanced healthcare infrastructure—continue to offset challenges related to cost and training gaps. As innovations accelerate and device safety improves, atherectomy is set to play a defining role in the future of cardiovascular care.
This market isn’t just growing—it’s evolving, modernizing, and reshaping how vascular disease is treated in the United States.