You’re reading the web edition of STAT’s Health Tech newsletter, our guide to how technology is transforming the life sciences. Sign up to get it delivered in your inbox every Tuesday and Thursday.
A tumultuous year at the Food and Drug Administration will be capped off at the agency’s devices center with the departure of two key leaders, just as regulators are sorting through challenges related to artificial intelligence and launching new initiatives on software as a medical device regulation.
Sources tell us Jessica Paulsen, a 15-year veteran of FDA and acting deputy director of its Digital Health Center of Excellence is leaving the agency. She’s been leading the center since last summer when the last acting head, SonjaFulmer, left FDA for Mayo Clinic. Fulmer took over for Troy Tazbaz who left in January to return to Oracle. The center’s work includes communicating with industry and developing guidances relevant to digital health. (FDA did not respond to a request for comment.)
Neuralink, Elon Musk’s frothy brain-computer interface company, poached David McMullen, director of FDA’s office of neurological and physical medicine devices, which is in charge of regulating Neuralink. McMullen spent three years atop the office and previously worked at the National Institute for Mental Health.
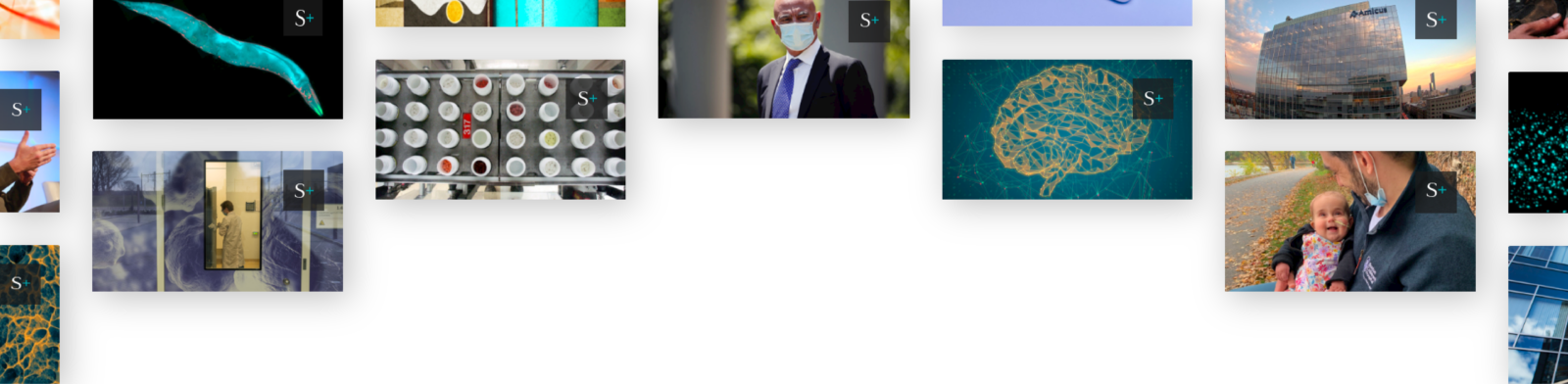
 David McMullen, director of FDA’s Office of Neurological and Physical Medicine Devices, and Jessica Paulsen, acting deputy director of the agency’s Digital Health Center of Excellence.
David McMullen, director of FDA’s Office of Neurological and Physical Medicine Devices, and Jessica Paulsen, acting deputy director of the agency’s Digital Health Center of Excellence.
Both Paulsen and McMullen were at the forefront of important conversations about the future of regulation. I grabbed the screenshot above of the two leaders from a video of last month’s Digital Health Advisory Committee meeting on generative AI-enabled mental health devices. Separately, McMullen’s office will have oversight of behavioral health devices under the FDA’s new TEMPO pilot.
New to me: As part of the funding package that reopened the government last month, lawmakers passed full-year 2026 funding for FDA. Buried within the Senate report accompanying the legislation, lawmakers direct FDA to, within 90 days, (February) report on its authorities to regulate AI medical devices, and within 180 days, (May) report on “the status of the FDA’s efforts regarding engagement on AI in drug development.”
The Government Accountability Office last week released a report on medical device recalls which found, among other things, that “insufficient staff limit FDA’s ability to conduct oversight activities.” In other words, the FDA already does not have enough staff to oversee medical devices and is losing key leadership at a time when new technology and initiatives may require additional horsepower.
The future of the mammogram
Applying AI to mammograms to help radiologists spot signs of breast cancer is increasingly common but researchers and AI companies want to apply new analyses to the routine screening tests to trigger more proactive care to prevent future cancers, heart attacks, and strokes. In one important breakthrough, the startup Clairity received FDA authorization for AI that offer a prediction of somone’s five-year breast cancer risk based on a mammogram alone.
STAT+ Exclusive Story
Already have an account? Log in
This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and news alerts.
Already have an account? Log in
Individual plans
Group plans
To read the rest of this story subscribe to STAT+.


