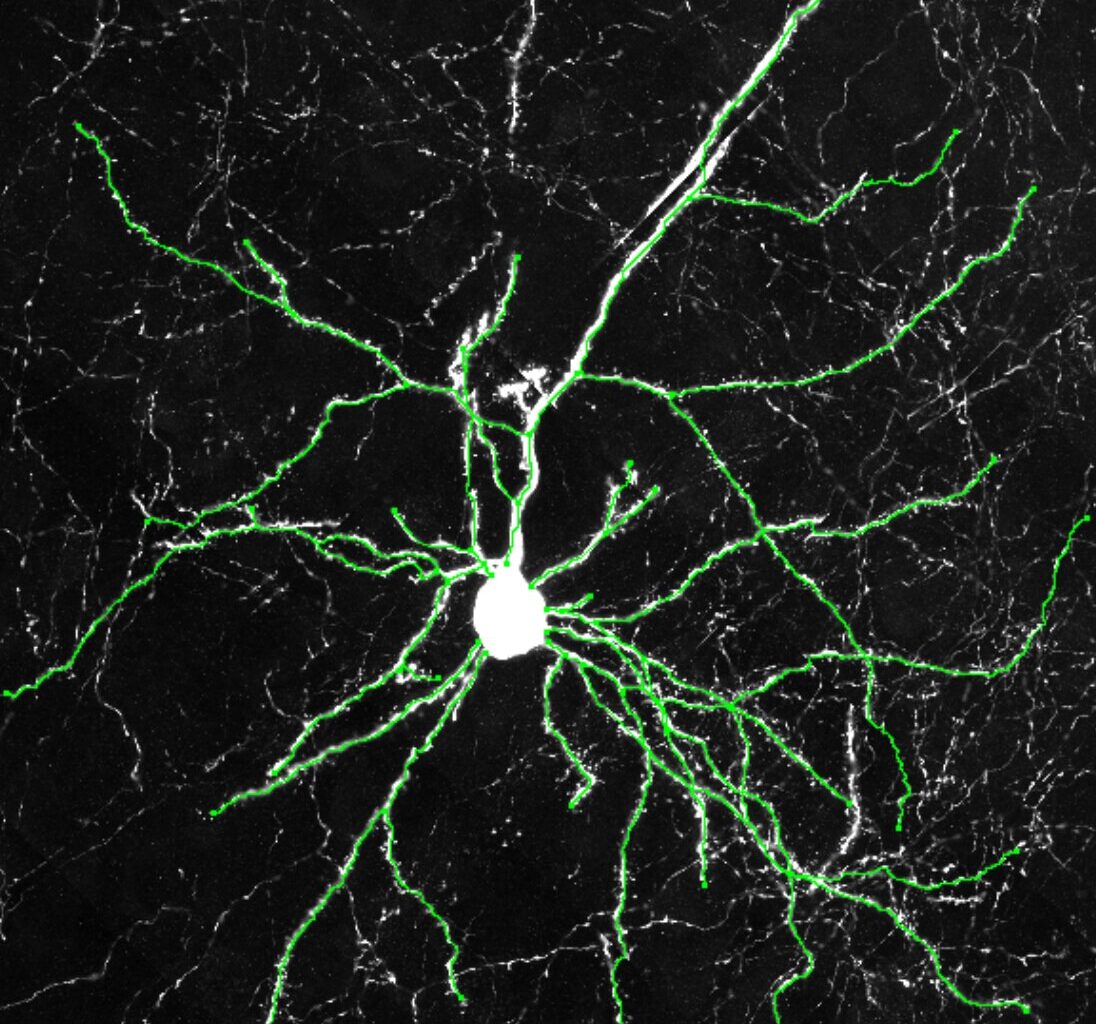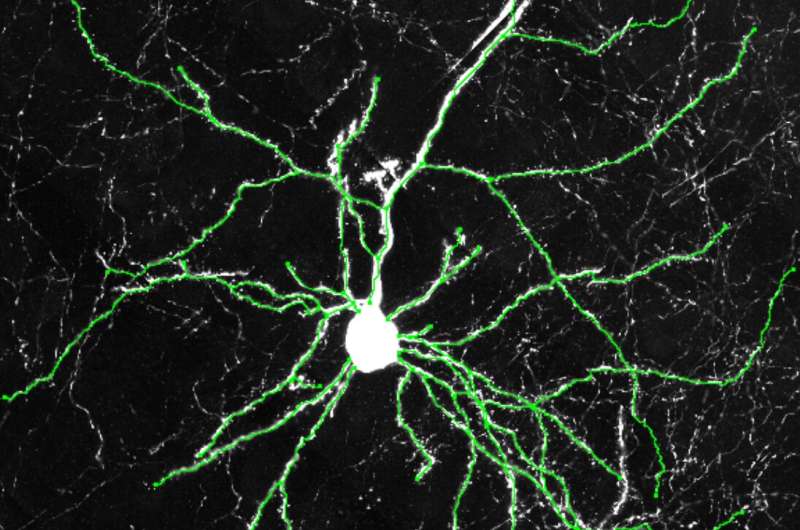
Example images of excitatory neurons from the cerebral cortexes of humanized mice showing how the neurons from the humanized mice grew more dendrites. Credit: Dr. Yuxiang Liu
Clock genes are a set of genes known to contribute to the regulation of the human body’s internal 24-hour cycle, also known as the circadian rhythm. One of these genes is the so-called CLOCK gene, a protein that regulates the activity of other genes, contributing to recurrent patterns of sleep and wakefulness.
Past findings suggest that this gene is also expressed in the neocortex, a brain region that supports important cognitive abilities, including reasoning, decision-making and the processing of language. However, the gene’s possible contribution to these specific brain functions remains poorly understood.
Researchers at UT Southwestern Medical Center recently carried out a study on genetically modified mice aimed at better understanding how the expression of the CLOCK gene in the human neocortex influences cognitive functions. Their findings, published in Nature Neuroscience, suggest that the gene plays a role in the formation of connections between neurons, which in turn influence mental and behavioral flexibility.
“Comparative genomic studies of the neocortex in humans and non-human primates have repeatedly identified human-specific upregulation of the CLOCK gene, suggesting that CLOCK may play a critical role in the evolution of the human brain’s advanced cognitive functions,” Genevieve Konopka, senior author of the paper, told Medical Xpress.
“Intriguingly, although CLOCK is a core circadian regulator, it exhibits distinct properties in the human neocortex: it is highly expressed in cortical neurons, lacks overt rhythmicity, and regulates a broad set of non-circadian genes. These findings from both evolutionary genomics and circadian biology converge to suggest novel, human-specific neuronal functions of CLOCK.”
To explore the possible contributions of the CLOCK gene beyond the regulation of the circadian rhythm, Konopka and her colleagues first created a humanized mouse model using genetic techniques. They specifically modified the expression of the gene in the brains of mice, so that it closely mirrored its expression in the human neocortex.
The expression patterns that they engineered in mice were so far only observed in humans. Therefore, the team’s hypothesis was that regions regulating the gene’s expression might have gone through specific changes during evolution.
“To test this hypothesis, we used recombineering to edit a human bacterial artificial chromosome (BAC) containing the full-length CLOCK gene along with its flanking regulatory elements,” explained Konopka. “This construct was used to generate a humanized CLOCK mouse model (HU).
“In standard learning and memory tasks, we observed no significant differences between HU and wild-type mice, possibly due to a ceiling effect. Therefore, we assessed cognitive flexibility using a set-shifting task, which demands higher-order, rule-based learning.”
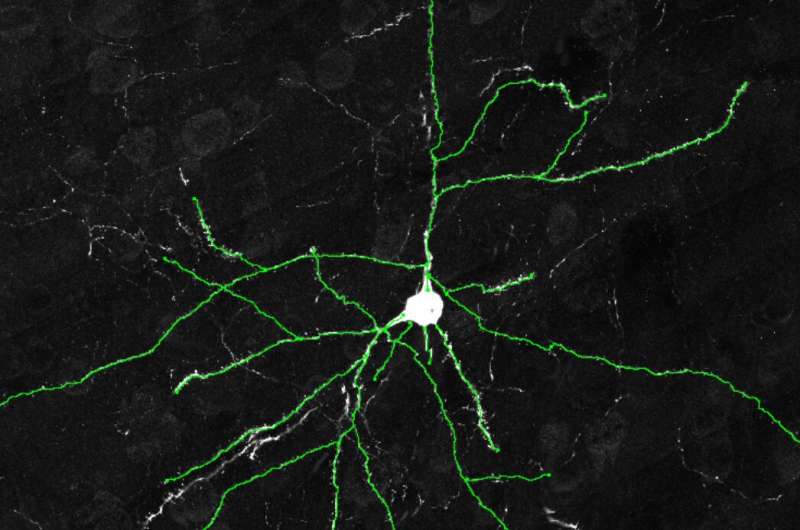
Example images of excitatory neurons from the cerebral cortexes of wildtype mice showing fewer dendrites. Credit: Dr. Yuxiang Liu
To determine when and in what brain regions or types of brain cells the CLOCK gene is active, the researchers studied the brains of the modified mice at different stages of development using a combination of experimental techniques. These included immunohistochemistry (IHC), a technique that relies on antibodies to detect specific proteins in sections of tissue, and single-nucleus RNA sequencing (snRNA-Seq), a high-resolution tool to measure the activity of genes in individual cell nuclei.
“To further identify neuronal functions regulated by human CLOCK, we examined neuronal cytoarchitecture and synaptic connectivity using confocal imaging and electrophysiological recordings,” said Konopka. “Finally, to validate our mouse findings in a human context, we employed CRISPR-edited human induced pluripotent stem cell (iPSC) models, confirming the dendritic and spine phenotypes associated with human CLOCK.”
Overall, Konopka and her colleagues found that human CLOCK regulates the expression of genes in neurons that contribute to the activation of other cells (i.e., excitatory neurons). They hypothesize that this process promotes the formation of more intricate neural networks, which are linked with greater cognitive flexibility.
The changes they observed in mice were mediated by the altered expression of the gene in the frontal cortex of the “humanized” animals. This suggests that they successfully altered the mice to mimic how CLOCK is expressed in the human neocortex.
“Our findings demonstrate that CLOCK has undergone evolutionary gains of extra-circadian function, and that these functions may contribute to human-specific brain specializations,” said Konopka. “This work makes several novel and important contributions to the understanding of CLOCK’s neural function and its role in human brain evolution.
“First, we developed both a humanized CLOCK mouse model and a CRISPR-mediated CLOCK knockout human iPSC model, which represent valuable resources for the broader scientific community. Second, we identified a previously unrecognized extra-circadian function of human CLOCK in excitatory neurons of the neocortex.”
This recent study is among the first to investigate the contribution of a gene known to support circadian rhythms to other brain functions. In the future, the findings gathered by Konopka and her colleagues could inspire other research groups to explore the extra-circadian functions of clock genes using humanized animal models.
“We also provide one of the first lines of evidence that a novel gain of function through altered spatiotemporal gene expression can underlie aspects of human brain evolution,” added Konopka.
“An intriguing finding from this study is the increased cell density, including both neurons and glia, in the neocortex of HU mice, suggesting that human CLOCK may influence neurogenesis during embryonic development. Future work will focus on characterizing the expression and function of human CLOCK in neural stem cells.”
Written for you by our author Ingrid Fadelli,
edited by Lisa Lock, and fact-checked and reviewed by Andrew Zinin—this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive.
If this reporting matters to you,
please consider a donation (especially monthly).
You’ll get an ad-free account as a thank-you.
More information:
Yuxiang Liu et al, Human CLOCK enhances neocortical function, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-01993-4
© 2025 Science X Network
Citation:
Human CLOCK gene enhances brain connectivity and mental flexibility in mice, study finds (2025, July 25)
retrieved 27 July 2025
from https://medicalxpress.com/news/2025-07-human-clock-gene-brain-mental.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.