 Credit: wildpixel / iStock / Getty Images Plus
Credit: wildpixel / iStock / Getty Images Plus
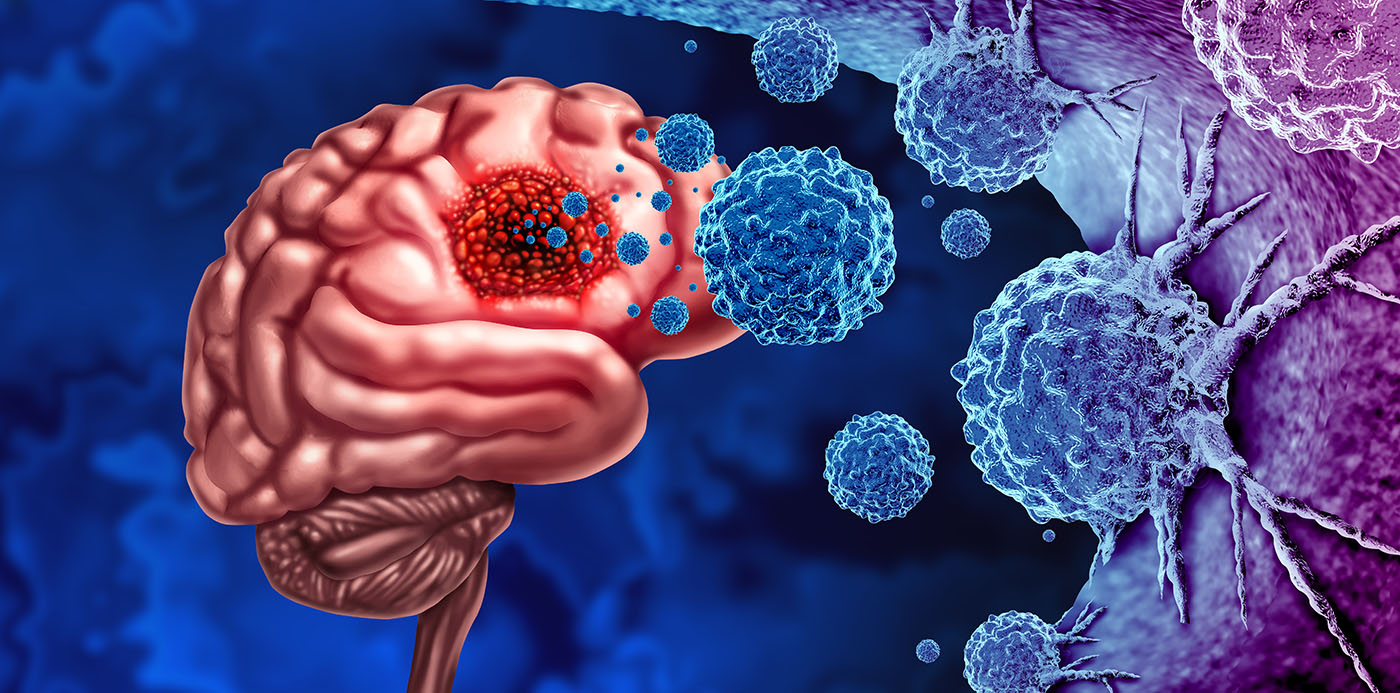
Gliomas are the most common primary brain tumor. Among all forms of glioma, glioblastoma is both the most aggressive and the most common, representing as many as 55% percent of all gliomas according to data from the National Institutes of Health.
The challenges to treating glioblastoma are many, which is part of why it so deadly. Glioma is often diagnosed at later stages of the disease. In addition, glioblastoma could be considered two diseases: one comprising a solid tumor that most often forms in the frontal or temporal lobes and the other an infiltrating element that coexists with normal brain cells. Treatment that involves surgically removing the tumor, therefore, cannot address the second element.
Currently an incurable cancer, most treatments focus on controlling the tumor to preserve a patient’s quality of life for as long as possible. The prevailing treatment regimen, often referred to as the Stupp protocol, has remained virtually unchanged for nearly 20 years and involves tumor resection followed by radiation therapy and chemotherapy with temozolomide. Progress to improve treatments has been slow due to factors like invasiveness, tumor heterogeneity, immunosuppressive characteristics of the tumor microenvironment, and a variety of resistance mechanisms to both chemo- and radiotherapy.
 Matija Snuderl, MD
Matija Snuderl, MD
Professor and Director
NYU Langone Medical Center
The frustration that accompanies the slow progress is palpable among researchers. “If ten years from now, I have to sit at a tumor board and hear ‘temozolomide and radiation,’ I’ll know I wasted my life,” said glioblastoma researcher Matija Snuderl, MD, a professor of pathology and director of molecular pathology and diagnostics at the NYU Langone Medical Center.
Recent research efforts leveraging improvements in sequencing technology, combined with machine learning and artificial intelligence (AI), are now accelerating advances in the development of new therapeutic modalities like immunotherapy, as well as in diagnostic approaches that can more accurately characterize disease subtypes. These advances perhaps foretell a future of targeted therapies for glioblastoma.
For instance, based on research at Snuderl’s NYU lab, and clinical validation completed in 2019 used a combined machine learning and genome-wide profiling approach to develop a DNA methylation-based system identifying epigenetic signatures to classify CNS and other tumors. Using this approach researchers have been able to accurately classify more than 180 different subtypes of CNS tumors including more than 10 distinct molecular entities that were previously lumped into a single GBM diagnosis.
“It has really changed the paradigm,” Snuderl said. “Because when I was a pathology fellow, all our diagnosis of glioma largely was based on hematoxylin and eosin slides, which is a technology from the 19th century. That’s all we had. Now we know that all these tumors that look alike are actually composed of multiple biologically, clinically, molecularly distinct entities. It’s the first time we have a better chance of finding new therapies.”
Insights from extrachromosomal DNA
Multi-omics studies are reshaping our understanding of a variety of diseases, including cancer. Having seen the understanding of other cancers shoot ahead, glioma and glioblastoma researchers are finally along for the ride.
In one such endeavor, researchers at City of Hope and collaborating institutions have constructed a genomic framework that maps, for the first time, how extrachromosomal DNA (ecDNA) shapes the spatial and molecular landscape of gliomas. By using spatial transcriptomics, tumor-normal DNA sequencing, and bulk RNA analysis, they demonstrated how ecDNA and tumor heterogeneity co-evolve, generating subclonal tumor regions with distinct oncogenic profiles. This research provides insight into the plasticity of glioma genomes and a way to better identify therapeutic targets.
 David Craig, PhD
David Craig, PhD
Professor and Chair
City of Hope
“We were seeing these ecDNAs—what used to be called double minutes—as far back as 2011,” said David Craig, PhD, professor and chair of the department of integrative translational sciences at City of Hope. “They form a circular plasmid-like structure outside the chromosome, often carrying hundreds of copies of oncogenes like EGFR. But what’s really interesting is they are not just passive carriers. They are actively changing the tumor’s microenvironment at the DNA level.”
Currently, the classification of gliomas relies on a spectrum of histological and molecular markers, like mutations in IDH1/2, TP53, and alterations in EGFR, ATRX, and chromosomal copy number states. Previous studies to characterize glioma heterogeneity have either lacked spatial resolution or did not integrate multi-omics datasets that could provide insights into the full scope of tumor evolution.
The new research by Craig and colleagues focused on a less-explored area of glioma genomics—the role of ecDNA. They specifically focused on double minute chromosomes that exist outside of the traditional chromosomal framework and amplify oncogenic drivers such as EGFR, MDM2, and MDM4. The investigators analyzed 11 gliomas spanning glioblastomas, astrocytomas, oligodendrogliomas, and diffuse midline gliomas, along with a replication cohort of six additional high-grade glioblastomas. Using spatial transcriptomics, they tracked how ecDNA-driven oncogenes and somatic loss of heterozygosity (LOH) events influence tumor cell behavior in different regions of the tumor microenvironment.
The team found that EGFR amplification, often observed on ecDNA, occurred in spatially distinct clusters within glioblastomas. These EGFR-high regions corresponded to hypoxic tumor zones, consistent with prior reports that hypoxia contributes to therapy resistance and poor prognosis.
“Wherever we saw the progression to hypoxia, we also saw double minutes—either separate or combined amplifications of EGFR and MDM2/4,” Craig said. “In one case, the tumor lost the entire chromosome 17, which carries TP53, and it had the same functional effect. That’s when we realized we were looking at two major pathways converging in these aggressive regions.”
Spatial transcriptomics uncovered this phenomenon in glioblastoma A1, revealing a subclone with EGFRvIII expression and complete LOH of chromosome 17 containing a TP53 mutation. The spatial alignment of EGFR amplification and TP53 allelic loss indicated that these genomic events may cooperate to form hyperproliferative regions, potentially setting the stage for aggressive progression or therapeutic resistance.
Analysis of other glioblastoma samples revealed amplification of MDM2 and MDM4 on ecDNA in separate tumor regions. These regulators inhibit the p53 tumor suppressor pathway, reinforcing the conclusion that ecDNA not only promotes oncogene amplification but also facilitates the inactivation of key tumor suppressors. In regions where both EGFR and MDM2/4 were amplified, cells appeared less reliant on hypoxia-driven angiogenic signaling, suggesting a shift toward more autonomous and proliferative behavior.
“This is about plasticity, how tumors can adapt and carve out these micro-niches,” Craig said. “The EGFR and TP53 pathways together seem to condition the cells to exclude immune infiltration, creating these protected cores where cells thrive under hypoxia.”
From a clinical and translational perspective, this research lays the groundwork for future studies to integrate spatial and genomic data with an eye toward developing more targeted therapies. Identifying tumor subregions that harbor both EGFR amplification and p53 pathway inactivation may help stratify patients for clinical studies. By mapping the distribution of ecDNA-driven oncogenes, oncologists may soon be able to identify regions of potential therapeutic resistance or recurrence.
Despite limitations that the authors noted, such as sequencing depth and resolution constraints inherent to current spatial technologies, they believe that their work provides an important model for applying integrative multi-omics to tumor biology. As these methods evolve and scale, the utility of spatially resolved tumor profiling will continue to improve for cancers like glioma. By elucidating how ecDNA and subclonal evolution shape the glioma microenvironment, researchers now have fertile ground to identify drug targets.
“We want to get where lung cancer is,” Craig noted. “Lung cancer has EGFR inhibitors that work. We’re working with the same genes in glioma and not getting the same results. That tells me we’re missing something. Understanding how ecDNA drives this plasticity is key to filling that gap.”
Insights from Hi-C
Recent advances in structural variant (SV) detection are redefining the molecular understanding and clinical management of gliomas, particularly glioblastoma, which is one of the most heterogeneous and therapeutically resistant forms of brain cancer. Building on his lab’s groundbreaking research in DNA methylation, Snuderl and team recently applied Hi-C chromatin conformation capture to formalin-fixed, paraffin-embedded (FFPE) tissue samples to better understand how cancers and gliomas develop. The team showed that Hi-C that could detect complex structural variants (SVs) at the DNA level, including rearrangements involving non-coding regulatory regions. This allowed the NYU researchers to identify tumor drivers that were previously not detectable via RNA next-generation sequencing (NGS) or other standard clinical panels.
The three-dimensional (3D) perspective provided by Hi-C can be combined with methylation and transcriptomic insights to form a triumvirate of analytical methods for the identification of new biomarkers. With Hi-C, tumors once considered genomically silent can now be interrogated for hidden drivers.
In a study analyzing 71 FFPE samples across ten solid tumor types, Hi-C showed 98% concordance with established clinical tests while uncovering additional SVs in cases previously classified as negative. Among these, one subset had rearrangements involving targetable biomarkers like NTRK and PD-L1, which were not expressed as fusion transcripts but comprised regulatory hijacking events detectable through 3D genome structure analysis. In glioblastoma, where co-amplified tyrosine kinase receptors and multiple subclonal populations coexist, such events are significant.
“If you just target EGFR, you would have this whole other clone that you cannot target,” said Snuderl, noting that this heterogeneity “is an inherent problem of gliomas.”
 Credit: gorodenkoff / iStock / Getty Images Plus
Credit: gorodenkoff / iStock / Getty Images Plus
Hi-C’s ability to detect gene rearrangements with noncoding regions is an important advance. “These rearrangements are completely invisible to all the clinical panels,” Snuderl said. The detection of ecDNA and chromothripsis through spatial analysis provides additional insight into the mechanistic drivers of therapy resistance, as also shown by the recent City of Hope research.
The heterogeneity of gliomas has made targeted therapy particularly challenging. Multiple subclones with different oncogenic alterations can exist within the same tumor, enabling clonal selection and resistance under selective pressure. Structural rearrangements involving non-coding DNA, metabolic divergence between pediatric and adult gliomas, and epigenetic plasticity further complicate treatment. “Considering glioblastoma one disease is the same as if you said that leukemia is one disease,” Snuderl said, while admitting that for years he was jealous of the tools that could be used to diagnose and classify different forms of leukemias and lung cancers. “I wished that one day we would be there, and now we are there. Molecular neuropathology has now outpaced probably all other fields in how well we can classify brain tumors.
“We sub-classified this disease to molecular minutiae in every possible way. I’m not saying we know exactly the significance of each of those subtypes, but we know that they are different.”
Snuderl is now hopeful that Hi-C can experience a relatively quick transition as a diagnostic tool. It requires fewer tissue slides than RNA NGS, is compatible with existing NGS infrastructure, and employs a standardized, single-pot chemistry workflow that can be automated, all of which should aid in future development. Additionally, Hi-C can be performed on FFPE samples up to 15 years old, expanding access to archival material that is critical to identifying biomarkers and drug targets.
“The idea was always to build it here (at NYU Langone) and then distribute it,” Snuderl said, which aligns with his broader vision of democratizing access to precision diagnostics by translating them into routine clinical care.
Sequential imaging to detect recurrence
Not all recent advances in glioma characterization have come from molecular analysis. A new study led by researchers at Mass General Brigham’s Artificial Intelligence in Medicine (AIM) Program leveraged deep learning models to analyze sequential magnetic resonance imaging (MRI) scans and showed that they could significantly improve the prediction of glioma recurrence in pediatric patients.
 Benjamin Kann, MD
Benjamin Kann, MD
Assistant Professor
Harvard Medical School
Using nearly 4,000 post-treatment MRI scans from 715 pediatric patients across three institutions, the research team built a self-supervised temporal deep learning tool that analyzed changes in brain scans over time. Unlike traditional AI models, which evaluate single imaging timepoints, their method taught the algorithm to recognize the chronological sequence of scans before training it to predict recurrence. “We hypothesized that AI could be trained to also analyze serial scans, but that this would require novel techniques,” said Benjamin Kann, MD, an assistant professor of radiation oncology at Harvard Medical School and part of the AIM Program. “Simply feeding a traditional AI tool multiple scans leads to poor performance, so we devised a strategy using a self-supervised learning technique called temporal learning.”
This approach substantially improved the accuracy of predicting disease recurrence, achieving 75–89% accuracy for identifying recurrence within one year of completing treatment. This was significantly better than the current rate of 50% accuracy achieved using single images. Improvements plateaued after incorporating four to six sequential scans, suggesting a practical threshold for future clinical use. The model’s design also mimics clinician reasoning by focusing on comparative changes over time, which could support greater trust in its outputs. “When we as radiologists or clinicians interpret scans in surveillance, we always compare the current scan to prior scans,” said Kann. “Our AI tool was designed in the same way.”
Another significant finding was that the model performed reliably across the spectrum of glioma subtypes, despite their heterogeneity. According to Kann, “Heterogeneity actually is a good thing in developing models like these, because it allows the model to learn different signals that lead to faster, or slower, recurrence.”
The study’s findings offer potential avenues for risk-adapted surveillance strategies and earlier therapeutic intervention. In future clinical trials, the team hopes to evaluate whether early treatment initiation for high-risk patients identified by the model improves recurrence-free survival. If validated, the tool may support reduced scan frequency for low-risk patients, alleviating the burden of long-term imaging for children and families.
Chris Anderson, a Maine native, has been a B2B editor for more than 25 years. He was the founding editor of Security Systems News and Drug Discovery News, and led the print launch and expanded coverage as editor in chief of Clinical OMICs, now named Inside Precision Medicine.