This narrative review explores the pathophysiology, clinical presentation, and management of visual aura in migraine, with a particular focus on CSD. A structured literature search was conducted across PubMed/MEDLINE and ScienceDirect to identify relevant publications. Search terms included combinations of “migraine”, “visual aura”, “scintillating scotoma”, “fortification spectra”, “cortical spreading depression”, “neuroimaging”, and “migraine treatment”, using boolean operators to refine results.
Studies published in English between 2000 and 2025 were considered. Inclusion criteria comprised original research articles, clinical trials, case reports, and narrative reviews addressing the visual manifestations of migraine and the underlying neurological mechanisms. Exclusion criteria included studies focused solely on migraine without aura, non-English publications, and articles without full-text access.
Article selection was conducted manually by reviewing titles and abstracts for relevance, followed by full-text screening. Additional sources were identified through reference mining of included studies. Thematic saturation was achieved when no new concepts emerged from additional articles.
Pathophysiology of visual aura in migraines
CSD is widely recognized as the leading scientific explanation for the pathophysiology underlying the propagation of visual aura experienced during a migraine attack. Clinical syndromic features pertaining to visual aura often manifests as transient visual disturbance in which individuals may observe flashing lights (photopsia) or zigzag patterns (scintillating scotomas) preceding the start of the migraine’s ‘headache phase’ [1]. CSD is characterized by a slowly-propagating wave of neuronal and glial depolarization across the cerebral cortex, leading to temporary suppression of cortical activity and changes in cerebrovascular circulatory blood flow [7]. It is hypothesized that CSD corresponds with the visual disturbances experienced peri-MA, thereby stipulated as the gold-standard of neurological basis for aura experienced in migraineurs.
As previously mentioned, CSD slowly propagates across the cerebral cortex via a wave of depolarization involving associated neurons and glia followed by a brief period of cellular depression. The CSD initiates with a localized wave of excitation that rapidly extends at a rate of approximately 2–5 mm per minute, encompassing the entirety of the cerebral cortices [8]. This depolarization is potentiated by an influx of calcium ions facilitating the release of excitatory neurotransmitters, such as glutamate. This extensive depolarization causes a transient depression in neuronal functioning and disruption in cortical activity, often directly proportional to the onset of MA [9]. Moreover, this wave of CSD is hypothesized to modify the normal functioning of the cerebrum, especially concentrating within the occipital cortex responsible for the processing of visual stimuli from the external environment. As CSD progresses through the occipital cortex, visual processing in associated brain parenchyma is affected, where visual symptoms like that of photopsia and scintillating scotoma ensue. The transient and self-limiting nature of CSD aligns with the typically brief duration of MA, commonly lasting between 5 and 60 min in symptomatology [10].
Functional imaging studies comprising functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have demonstrated transient changes within cerebrovascular blood flow and cortical activity, consistent with CSD in patients experiencing MA. Hadjikhani and colleagues employed fMRI to demonstrate said characteristic patterns of cerebrovascular blood flow in migraineurs, beginning in the occipital cortex and spreading anteriorly across the cerebrum, mirroring the progression of CSD [11]. Firstly, a short phase of increased cerebrovascular blood flow is exhibited, briefly followed by a prolonged phase of reduced vascular compliance and hypoperfusion. This pattern of altered perfusion corresponds to cortical regions affected during migraine aura, reinforcing the hypothesis that the changes in cerebrovascular blood flow and neuronal excitability that accompany CSD constitutes as an fundamental component of MA pathophysiology.
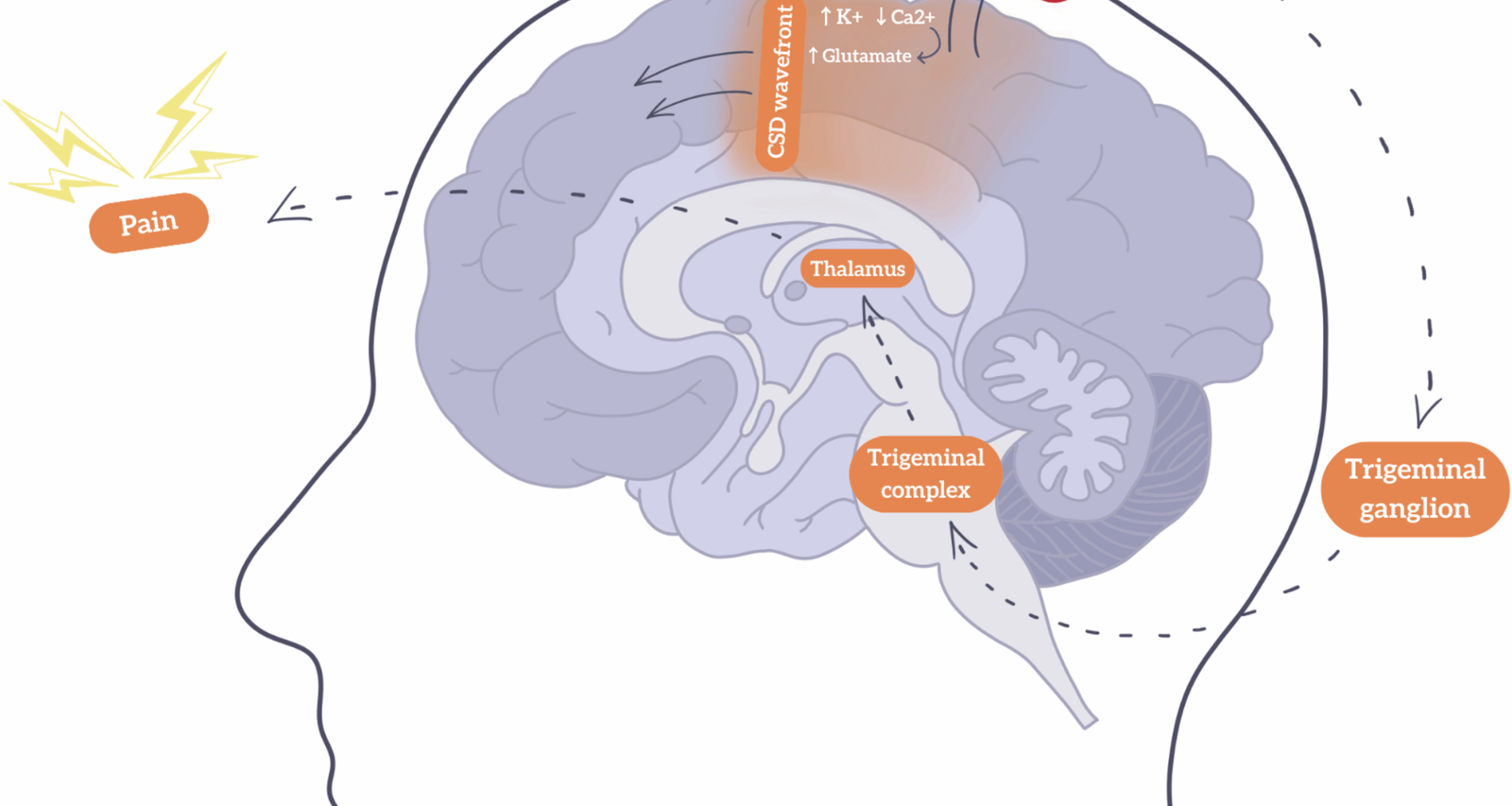
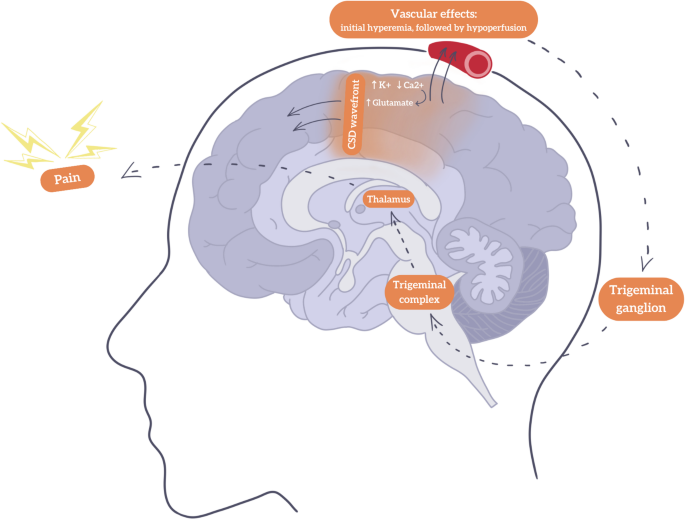
Ion channels resident to the central nervous system responsible for the influx and efflux of potassium and calcium, as well as excitatory neurotransmitters such as glutamate, play a focal role in the initiation and propagation of CSD. Strongly correlated with an increase in levels of extracellular potassium and intracellular calcium levels thereby stimulating subsequent glutamate release is thought to initiate CSD [12]. An upsurge in glutamate facilitates an increase in cortical excitability, promoting depolarization across the cerebral cortex. In conjunction with the influx and efflux of said ions through neurons and glia, global brain depolarization generates a positive feedback loop where resulting sustainment of depolarized wave activity ensues. Furthermore, N-methyl-D-aspartate (NMDA) receptors and voltage-gated calcium channels may also modulate neuronal excitability, thereby determining the speed and intensity of CSD [13]. The CSD mechanism is illustrated in Fig. 1, showcasing how the CSD initiates a cascade of neurovascular events that ultimately activate the trigeminovascular system, a key pathway in migraine pathophysiology.
Schematic representation of cortical spreading depression (CSD) and headache in migraine with aura. The CSD wavefront (↑K⁺, ↓Ca²⁺, ↑glutamate) spreads across the cortex, leading to vascular changes, initial increase in blood flow (hyperemia) followed by reduced perfusion (hypoperfusion), contributing to aura symptoms. This activates the trigeminovascular system via the trigeminal ganglion and complex, which are involved in transmitting pain signals from the meninges and cranial blood vessels to the brainstem and cortex. This then relays nociceptive signals to the thalamus and results in pain perception. Arrows indicate the direction of signal propagation
Types of migraine visual disturbances
Visual aura in migraine constitutes a variety of symptomatology. In a systematic review done by Viana et al. 30 different basic visual symptoms linked to migraine aura were found. These symptoms fall into three main categories, according to the visual aura table developed by the International Headache Society (IHS): positive phenomena (such as; scintillating scotomas, zigzag lines, or fortification spectra), negative phenomena (such as; visual field loss or temporary blindness) and distorted visual perception (such as; visual tilting or metamorphopsia) [5, 14].
According to Viana et al. in their multicentre, cross-sectional study, 60% of individuals reported bilateral visual field engagement during MA episodes, which was a topic of discussion in previous clinical investigations [5]. Other than blurred vision, which was reported by women more often than by men, the study identified no differences in the distribution of recognized elementary visual symptoms (EVS) among study sites, gender, or age groups [5].
Scintillating scotomas are among the most frequently reported neuro-ophthalmologic symptoms. Because of their distinctive flickering or zigzag patterns, they are usually categorized as a positive phenomenon. However, some authors describe it as a mixed phenomenon since it also affects the central or peripheral area of vision, causing a blind spot, which is considered under negative phenomena [1, 14, 15]. The development of scotoma and scintillation in the visual field is thought to be the consequence of occipital lobe neuronal dysfunctionality. This may be linked to waves of unparalleled brain activity, a feature most consistent with CSD [15].
Another characteristic visual aura in MA is teichopsia, also known as the fortification spectrum, described as a complex pattern of zigzagging, flickering lines that are classically irrespective of color, but occasionally color-dense. These lines of light typically begin as a tiny grey patch close to the center of vision, superseded by a gradual lateralization of light display. The growth in central patched light produces a temporary blind spot, possessing jagged, shimmering edges that last as the aura takes shape [1, 16, 17].
Often used interchangeably with scintillating scotomas, teichopsia refers especially to the initial zigzag visual pattern, whereas scintillating scotomas commonly describe the later phase that includes the development of a blind spot.
Additionally, frequently reported simple visual symptoms in different studies were foggy or blurred vision, bright light (phosphenes), small white dots referred to as ‘falling stars’, micropsia, macropsia, focal visual field defects, and hemianopia are further examples of simple visual patterns observed in MA that may be singular in appearance or in syndromic combination [5, 14, 17]. Table 1 summarizes the different types of visual disturbance that may occur during MA.
Table 1 Different types of visual disturbances that occur during migraine aura
For instance, in a study that employed flicker perimetry to evaluate the visual field, individuals known to have migraines frequently exhibited visual anomalies [18]. Two primary patterns of visual field loss were observed: localized deficits, such as scotoma, and generalized sensitivity loss across the visual field [6]. In addition, there have been reports of prolonged homonymous hemianopia linked to migraine [19]. In rare cases, migraine is credited for intermittent and full homonymous hemianopia [19].
Clinical findings suggest that the involvement of the occipital cortex in patients with migraine, reduction in cerebrovascular blood flow, and CSD generated coincides with a wave of global depolarization maturing posteriorly and disseminating anteriorly [19]. Substantial evidence from MRI data in migraineurs supports such a theory, where visual auras are indicative of global disseminating neural excitation that gradually propagates throughout the visual cortex, causing a brief period of neuronal depression that results in the development of a temporary scintillating scotoma [20]. Moreover, one study elucidated that individuals who developed homonymous hemianopia or scotoma at the time of the aura also suffered a prolonged but temporary suppression of their respective visual fields [20].
Studies have shown that the brain’s serotonin receptors possess different binding affinities for the licensed migraine medication and 5-hydroxytryptamine (5-HT) agonist, sumatriptan. This clarifies the role these receptors play in migraine development, particularly in visual aura. Although the globus pallidus, periaqueductal grey, amygdala, and entorhinal, frontal, and temporal cortices also display notable serotonergic activity, the visual cortex occupies the highest binding affinity. The paramount importance of the visual cortex in the processes that underlie migraine symptomatology is indicated by such visual patterns [21].
Moreover, migraineurs have reported hypersensitivity to color perception. Atypical reactions to red and blue have been seen during color perimetry. Such findings, which more often involve heightened color sensitivity or occasionally refer to conditions like achromatopsia, imply that individuals with migraine-related visual perturbance may have underlying pathologies of color processing [17]. Many shapes, including zigzag patterns, dots, lines, geometric figures, and bean-like formations, were described by participants who had colored EVS. Shades of grey, white, black, and silver were the colors most frequently stated [5]. Additionally, metamorphopsia occurs when individuals or objects appear ‘bent’ or ‘twisted’. While half of the observed facial characteristics appear to migrate upward or downward, others may exhibit “monstrous faces” as a result [17].
Diagnostic approaches in migraine-related visual aura
Among subtypes of migraine, visual aura is the most common, presenting with transient visual disturbances that often cause diagnostic ambiguity due to its symptomatic overlap with other neuro-ophthalmic conditions. The diagnostic approach to migraine-related visual aura involves a structured, multimodal assessment to differentiate said pathology from other neurological conditions. Table 2 summarizes the diagnostic steps in which the evaluation of a MA patient is conducted.
Clinical assessment
Clinical evaluation begins with a detailed and comprehensive history, critical for differentiating migraine-related visual aura from other conditions sharing similar syndromic feature. A thorough history enables clinicians to determine the specifics attributed to the aura described, including its disease nature (e.g., scintillating scotoma, fortification spectrum), onset, progression, and duration. Symptoms pertaining to MA are typically of gradual onset, developing over a period of five to twenty minutes, with symptom-free resolution coinciding within one hour. These clinical manifestations are fundamental when gauging a diagnosis of migraine [22].
To better understand the frequency, pattern, and triggers of aura episodes, patients are encouraged to retain a ‘headache diary’ [23]. The documentation of specific aura features and their temporal relationship with cephalgia provides key insights concerning unique symptoms and light patterns experienced with migraines. Headache diaries, when reviewed systematically, aid clinicians to observe recurring manifestations of aura, distinguish atypical presentations of aura, and monitor treatment response. In addition to headache diaries, structured patient interviews allow healthcare providers to assess subjective effects of visual aura qualitatively. Visual auras are typically described by patients as ‘flashing lights’, ‘zigzag lines’, or ‘blind spots’ [24]. Identifying the gradual onset of MA with comprehension of the most stereotyped patterns of visual phenomena is crucial as these may distinguish MA from more acute, insidious symptoms seen in conditions like transient ischemic attack (TIA) or ischemic stroke.
Imaging techniques
While a diagnosis of MA primarily relies on clinical criteria, neuroimaging may offer additional insight, particularly in atypical cases or when features of aura suggest other pathologies.
Our understanding of cortical dynamics observed episodically during an episode of MA may be visualised with fMRI studies. Such imaging has revealed waves of cortical activation followed by inhibition within the remits of the occipital cortex peri-MA, parallel to the phenomenon of CSD [11]. It is believed that this global dissemination of neuronal depolarization correlates with the visual disturbances experienced during an episode of MA. Appreciating such a phenomenon in neuro-ophthalmology aids one’s comprehension of pathophysiological basis, even though fMRI is primarily employed as a research apparatus due to ecological limitations in the modern clinical environment.
Amongst other neuroimaging lies PET as a modality, where knowledge pertaining to alterations in blood circulation within the cerebrocortices during episodes of MA revealed hypoperfusion in affected regions via CSD [25]. These changes in cerebrovascular blood flow during migraine aura are typically not associated with infarction, an essential clinical syndromic feature when distinguishing differential diagnoses from migraine, like that of TIA or ischemic stroke, where hypoperfusion may precipitate permanent damage to brain parenchyma if left untreated.
Electroencephalography (EEG) utility in localizing aura is limited due to its low spatial resolution. However, EEG may be employed to monitor disruption in cortical excitability during episodes of MA. Studies have shown altered excitability in the visual cortex peri-MA, supporting the role of cortical hyperresponsiveness in aura pathogenesis [26]. Although EEG is not routinely utilized for MA diagnostics, it may assist in research or when differentiating migraine from epilepsy in cases where the two conditions co-exist.
Differential diagnosis
Differentiating migraine-related visual aura from other conditions that causes transient visual disturbance is a critical component of the diagnostic process.
TIA, epilepsy (particularly that of occipital lobe seizures), and other neuro-ophthalmic disorders mimic visual aura [27]. TIA usually presents as an acute pathology which may exhibit focal neurological deficits not observed in MA. To distinguish MA from other ‘doppelganger’ syndromes, clinicians should consider the temporal characteristics of aura, attributed to a period of symptomatology lasting less than 60 min alongside accompanying sensorimotor deficit and altered consciousness. TIA may present in conjunction with hemiparesis or aphasia, and occipital lobe seizures may exhibit sudden-onset visual perturbance with convulsive features [28].
Table 2 Diagnostic steps in evaluating a migraine patient with visual auraManagement and treatment strategies for migraine with visual aura
There exists today a myriad of therapeutic modalities available for migraine with visual aura. Modern pharmaceutical agents available by prescription or over the counter may be divided into two main groups: preventive and abortive. Preventative therapies concentrate on reducing the frequency and severity of migraine episodes by targeting the underlying neural pathophysiology. On the contrary, abortive therapies are administered when managing the symptoms of an acute migraine attack [29]. These treatments have been summarized in Table 3. Preventative therapies for migraine with visual aura include beta-blockers (e.g., propranolol), calcium-channel blockers (e.g., verapamil), and anticonvulsants (e.g., topiramate). Beta-blockers, metoprolol and propranolol, function by decreasing the excitability of the cerebral cortex to prevent the initiation of CSD. A meta-analysis by Jackson et al. comprising 108 randomized controlled trials shown propranolol to be an effective therapeutic in decreasing the number of migraine headache episodes experienced by patients on a monthly basis, reducing the number of analgesics required when ameliorating cephalgia severity and duration [30]. Propranolol reduced episodic migraine headaches by 1.5 headaches/month at 8 weeks (95% CI: −2.3–0.65) and increased the likelihood of achieving a ≥ 50% reduction in headache frequency (RR: 1.4, 95% CI: 1.1–1.7) compared to placebo. For chronic migraine, propranolol was more likely to reduce headaches by at least 50% (RR: 2.0, 95% CI: 1.0–4.3), suggesting even greater efficacy in more persistent cases [30].
Table 3 Treatment options for migraine with aura, including both Pharmacological and non-pharmacological approaches
Calcium channel blockers, such as the centrally acting cardiac non-dihydropyridine verapamil, demonstrate therapeutic efficacy as a migraine preventative. These medications modulate the influx of calcium ions, thereby stabilizing the excitability of neurons and decreasing the susceptibility to CSD [31]. Anticonvulsants, specifically topiramate and sodium valproate, are also frequently administered as preventative agents. Topiramate has many pharmacological mechanisms of actions, including the modulation of sodium channels and enhancement of gamma-aminobutyric acid (GABA) activity which functions as an inhibitory neurotransmitter [31]. The most widely used group of abortive therapies are triptans, which function as serotonin (5-HT) receptor agonists. For example, sumatriptan is effective in providing relief from migraine headaches via cerebrovascular vasoconstriction through the 5-HT1B/1D receptors and inhibition of pro-inflammatory neuropeptides [30]. Nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen and naproxen, are also utilized as abortives, where lead to a decrease in inflammation and pain through the inhibition of cyclooxygenase (COX) enzymes, possessing a role in pro-inflammatory mediator production [33]. Although both triptans and NSAIDs are effective as an abortive therapy of migraine headache, their role in management of aura is limited.
Non-pharmacological approaches are an important aspect of managing MA, complementing medical therapies effectively. Lifestyle modifications, including dietary adjustments, stress management, and consistent sleep hygiene have demonstrated efficacy in reducing episodes of aura [36]. A regular sleep schedule with minimal disruption is essential in maintaining proper sleep hygiene, leading to stabilization of neurological function and a potential decrease in aura susceptibility. Stress management techniques include progressive muscle relaxation and meditation which aid to decrease aura frequency by dampening the activation of the sympathetic nervous system. Furthermore, behavioral therapies, such as cognitive-behavioral therapy (CBT) and biofeedback, offer additional support in migraine control [37]. CBT provides patients an opportunity to identify and modify stress-inducing thought patterns whereas biofeedback uses real-time monitoring of physiological parameters (e.g., muscle tension, heart rate, blood pressure) to help patients gain control over their bodily homeostasis [38].
In recent years, there have been emerging treatment modalities for MA. An example is that of neurostimulation devices, including transcranial magnetic stimulation (TMS). TMS functions by applying magnetic pulsation to the scalp of a migraineur, modulating neuronal activity in the cerebral cortex, potentially inhibiting the propagation of CSD [10]. It has been suggested that TMS may improve the frequency and severity of migraines while also reduce symptoms of aura. TMS has shown promise in acute migraine relief (OR: 2.28) and reducing attack frequency (up to 7.5 fewer migraines per month), with associated improvements in headache days, medication use, and Headache Impact Test (HIT-6) questionnaire scores [34]. One other category of emerging therapeutics comprises calcitonin gene-related peptide (CGRP) small molecule antagonists Atogepant, Rimegepant, and Zavegepant alongside the human monoclonal antibodies Erenumab and Fremanezumab which actively bind to the CGRP receptor [37, 38]. These medications function by blocking the activity of CGRP involved in cerebrovascular vasodilation and nociception peri-migraine episode [35]. Anti-CGRP monoclonal antibodies significantly reduced monthly headache and migraine days (p <.0001), drug intake, and disability scores, such as migraine disability status scale (MIDAS) and HIT-6, with some patients converting from chronic to episodic migraine by the first trimester of treatment. However, their effect on aura frequency remains limited [35].
Thus, managing MA necessitates a multifaceted approach involving conservative measures like that of lifestyle modification, abortive and preventative therapies, and emerging experimental treatment. Ongoing research into neurostimulation and the use of CGRP inhibitors holds promise for more effective, targeted management strategies that address both the cephalgia and aura experienced by migraineurs.
Challenges and gaps in Understanding migraine-related visual aura
Various theories, including neural, trigeminal, and vascular propositions, have attempted to explain migraine pathophysiology. Nevertheless, there remains much contradicting evidence basis, and no theory offers a comprehensive vindication behind the disease pathophysiology of migraine. Such uncertainty appears as a dearth in the literature and current research pertaining to migraine management [39]. Additionally, one great challenge for patients with migraine is the limitation and effectiveness of modern treatment modalities. In a qualitative interview study, migraineurs reported that even though a plethora of drugs exist on the market that aims to reduce acute migraine attacks, there persists real limitations in prophylactic and aura-preventive therapies. Moreover, participants expressed dissatisfaction with the efficacy of current treatments, citing issues with tolerability and adherence due to adverse drug reactions experienced [40]. Furthermore, other treatment methodologies consisting of CGRP human monoclonal antibody, gepants, and single-pulse TMS have produced mercurial clinical outcomes [40, 41].
The third version of the International Classification of Headache Disorders (ICHD-3) offers substantial information concerning the clinical characteristics of aura. However, the data collated from observational studies is frequently considered to be insufficient for a comprehensive theoretical rationalization of disease pathophysiology [42]. This thorough evaluation of the characteristics circumventing aura in migraineurs has been hindered by the exclusion of particular aura symptoms and small sample sizes leading to exponentiated amounts of type 2 error [42]. Moreover, correlations and assumptions made between the interrelations of migraine cephalalgia and aura as a direct comparison is often arduous given differences in study demographics and methodologies. To enable accurate diagnoses in clinical settings and improve disease characterization, this gap must be filled.
There exists a limit in the number of studies that focus on migraine without aura (MWA), despite MWA being the most common surmounting 70–85% of all cases of migraine. Compared to MA, its pathophysiology is still unclear. From this perspective, future studies should examine the pathophysiology of MWA [43]. Lastly, to garner a deeper understanding of the biochemical processes that underlie migraine, more research is warranted. New perspectives on disease prevention, the redesigning of care pathways, multidisciplinary management planning, and the adoption of individualized therapeutic approaches may be ascertained through the identification of critical biopsychosocial components alongside comprehending the pathophysiological predeterminants [44].
Pathophysiological and neuroimaging advances
CSD remains the principal neurophysiological mechanism underlying migraine aura, particularly visual aura. This review builds upon prior literature by emphasizing the evolving understanding of CSD’s ionic and metabolic underpinnings. Specifically, recent studies have identified modulators such as 20-HETE accumulation and ion-channel dysregulation that influence post-CSD hypoperfusion and excitability, offering deeper mechanistic insights into aura pathogenesis beyond earlier summaries [8, 9].
Moreover, neuroimaging has significantly advanced our ability to visualize aura dynamics in vivo. High-resolution fMRI in migraineurs with aura demonstrates a propagating wave of occipital activation followed by brief cortical inhibition, mirroring the CSD pattern and aligning with the subjective progression of aura symptoms [11]. Additional modalities, including arterial-spin labeling and 7 T MRI, now allow for unprecedented detail in tracking cortical and vascular responses during aura, which were previously inaccessible in older reviews. This integration of advanced imaging helps substantiate CSD as both a clinical and physiological substrate for migraine aura.
Emerging treatment strategies
New therapeutic modalities are targeting both the headache and aura phases of migraine. TMS, a non-invasive neuromodulation technique, may interrupt the propagation of CSD and abort aura symptoms if applied early. Systematic reviews report that single-pulse TMS significantly increases acute relief (OR: 2.28) and reduces monthly attack frequency by up to 7.5 episodes [34]. These effects suggest that TMS may offer dual benefits for both headache and aura components.
CGRP pathway inhibitors, including monoclonal antibodies (e.g., erenumab) and small-molecule gepants (e.g., rimegepant), have also shown efficacy in reducing migraine frequency and disability scores [35]. However, current evidence indicates their impact on aura symptoms remains limited and variable. This review highlights the distinction between general migraine control and aura-specific efficacy, which is an area requiring further investigation.
Emergency evaluation and red flags
While migraine aura is typically benign, certain features warrant urgent evaluation to exclude serious neurologic conditions such as TIA or ischemic stroke. Classic aura symptoms progress gradually over 5–20 min and resolve within one hour [20]. In contrast, sudden-onset or prolonged (> 60 min) visual disturbances are atypical and should prompt neuroimaging.
Clinicians should also be alert to red flags, based on the ICHD-3 which includes [22]:
Persistent negative visual symptoms (e.g., homonymous hemianopia or vision loss).
New-onset aura in patients over 50 years of age.
Aura with motor, brainstem, or sensory features (e.g., dysarthria, weakness).
Vascular risk factors such as hypertension or atrial fibrillation.
These presentations deviate from the classic migraine pattern and necessitate prompt neurologic consultation and imaging (CT/MRI).
Limitations
As a narrative review, this manuscript is limited by the inherent subjectivity in literature selection and synthesis. No systematic search or meta-analysis was performed. The included studies span a wide range of methodologies, populations, and outcome definitions, which limits generalizability. Additionally, many of the referenced trials focus on headache outcomes, with aura-specific effects analyzed secondarily or not at all. Prospective, controlled studies investigating aura as a primary endpoint are scarce, especially for newer therapies such as TMS or CGRP inhibitors. Thus, while this review summarizes the current state of the field, its findings should be interpreted within the context of these limitations and the heterogeneity of existing data.
Future directions and recommendations
Research into MA increasingly focuses on localizing genetic markers that may indicate susceptibility. Familial clustering of migraine cases suggests a genetic predisposition, particularly in migraine with aura (MA), where epidemiological studies indicate a higher genetic contribution compared to migraine without aura [45]. However, despite this strong heritability, genome-wide association studies (GWAS) have identified only one significant locus (near MTDH and PGCP) specific to MA, suggesting shared genetic mechanisms between MA and other migraine subtypes [45]. Identifying more specific genetic markers may lead to earlier risk assessment and personalized preventative strategies. Longitudinal studies exploring gene expression patterns pre-, peri-, and post-aura episodes may offer valuable insights into the pathophysiological mechanisms pertaining to aura onset. Additionally, advancements in neuroimaging could enhance our understanding of migraine pathophysiology. Improved neuroimaging apparatus, for instance, may allow real-time visualization of CSD dynamics with greater resolution, shedding light on its role in aura onset and potentially guiding new interventions for early aura symptomatology [11]. Neuroimaging may also serve as a diagnostic aid when migraine aura presents atypically, if available clinically.
Despite these advancements, current targeted therapies for MA are limited, and loci of therapeutic focus on ‘headache phase’ alone is not substantial contemporaneously. Developing innovative methods of treatment that address the underlying disease pathology at hand is vital, and localizing the pathways in which CSD disseminates offers a promising direction. As CSD is considered the neural basis of MA, interventions that disrupt this process may prevent or alleviate symptoms of aura [24]. Pharmaceutical efforts that modulate ion channels and glutamate release are under investigation and hold potential. Non-invasive neurostimulation, including TMS and vagus nerve stimulation (VNS), also presents an encouraging therapeutic avenue, displaying efficacy in reducing migraine frequency, duration, and severity [46]. Despite previous inconsistencies within clinical outcome, future studies concentrating on patients with MA may help establish modalities of prophylaxes specifically targeting aura management as well as implementing individualization with migraine management.
A comprehensive understanding of MA will likely benefit from a multidisciplinary approach involving neurologists, ophthalmologists, and neuroimaging specialists. Since visual aura may mimic symptoms of other neuro-ophthalmic conditions, interdisciplinary collaboration is essential for accurate diagnosis and effective management. Regular case reviews and shared neuroimaging resources could further enhance diagnostic accuracy [27].
Clinicians should begin with a detailed patient history, focusing on aura characteristics and their relationship to headache. Aura without headache is often missed, underscoring the need for careful evaluation [14]. Patient education on trigger management and lifestyle modifications (stress reduction, sleep hygiene) is essential [14]. The use of headache diaries, as recommended by the ICHD-3, can improve diagnostic accuracy [22].
Diagnostic workup should include neurological examination and neuroimaging when needed. For atypical cases, EEG may help differentiate migraine from epilepsy [47]. Prophylactic options like beta-blockers or antiepileptics may be considered for frequent episodes, while NSAIDs can help with aura-associated headache [14]. Regular follow-up is advised, with multidisciplinary care for complex cases. Beyond individual management, it is crucial to acknowledge that systemic disparities in neurological care, particularly in low- and middle-income countries, continue to hinder equitable access to diagnosis and treatment. Addressing these gaps requires global collaboration, resource investment, and research that centers the needs of underserved populations [48].