Explanation for the choice of comparators {6b}
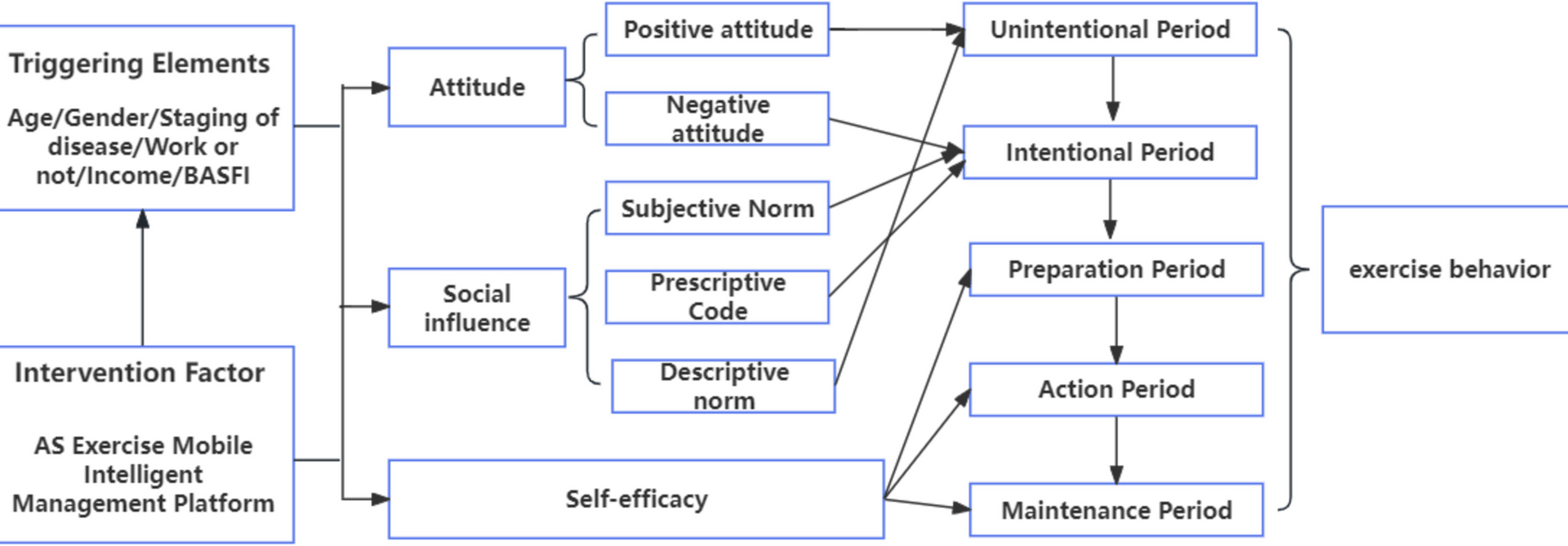
The intervention group is managed by “Exercise Management Mode based on ASE model (EMM-ASE)”. First of all, according to the exercise plan proposed by specialty doctors, participants in the intervention group will be asked to answer a question of “During the past 6 months, have you followed the exercise plan recommended by your doctor?” to evaluate their exercise behavioral intention. If the answer is “I cannot do it and I have no intention to do it in the future”, it means “precontemplation stage”. “It cannot be done and I intend to implement it within the next six months” means “contemplation stage”, “It cannot be done, but I intend to implement it within the next 1 month” means “preparation stage”, “It can be done, but it has lasted less than 6 months” indicates “action stage”, and “I have persisted for more than 6 months” indicates “maintenance stage”. Then, different behavior management strategies will be implemented according to the intention stage, as shown in Table 1.
Table 1 Intervention implementation plan of Exercise Management Mode based on ASE modelIntervention description {11a}
(1) Log-in system: Specialty nurses will issue the “AS Exercise Mobile Intelligent Management Platform User Manual” to guide patients in using the platform and assist them in logging into the network broadcast exercise system.
(2) Exercise behavior and related factor assessment: Patients enter the “Exercise Behavior Assessment Module” and input corresponding information for each assessment item displayed in the system. The system will automatically assess and form the assessment results, which will be stored in the system’s background as one of the bases for the management procedure.
(3) Exercise behavior management: As listed in Table 1. Among them, the network broadcast exercises are provided on the public network broadcast platform, and the full-time rehabilitation specialist nurses deliver the class online. Patients can choose the appropriate time according to their own exercise plan to participate in exercise activity simultaneously.
(4) Intervention feedback: Specialty nurses will review the patient’s exercise behavior feedbackingly reported by the system every week and further handle patients with poor exercise compliance. Firstly, they will communicate with patients through network or phone to understand issues and difficulties during exercise, and provide online guidance through specialty doctors or nurses. When online guidance is not sufficient, refer patients for clinical visits, and specialty nurses will be responsible for referring them to the relevant personnel. For patients who have difficulty moving or are unable to travel, refer them to community medical staff for home visits.
(5) Stage evaluation: Conduct a monthly re-evaluation of the stage of behavioral intention and adjust the intervention content accordingly.
Criteria for discontinuing or modifying allocated interventions {11b}
(1) Patients are lost to follow-up for any reason during the study;
(2) Patients experience severe complications or adverse events necessitating their withdrawal from the study.
Strategies to improve adherence to interventions {11c}
First, we plan to establish a core research group. The Guangdong Rheumatology and Immunology Specialized Alliance will lead the establishment of the core research group, which will mainly consist of specialty doctors, nurses and research assistants.
The core research group will develop a unified research plan, including the selection of research subjects, grouping, intervention methods, data collection methods and provide training to all members of the research groups. Also, the “AS Exercise Mobile Intelligent Management Platform User Manual,” which will be divided into medical care and patient versions will be wrote by core research group, and provide training to all members of the research groups.
Relevant concomitant care permitted or prohibited during the trial {11d}
During the entire trial, both the original treatment regimen and the adjusted treatment regimen (including drug therapy and physical therapy) are permitted, and details of these treatments will be recorded. However, exercises or labor that may increase patient fatigue, cause exhaustion, or result in injuries (such as excessive fatigue, obvious shortness of breath, difficulty breathing, joint sprains, muscle strains, and aggravated pain) are not recommended.
Provisions for post-trial care {30}
The exercise methods in the protocol are all measures recommended by the guidelines. Patients may experience normal post—exercise reactions, such as moderate fatigue and mild muscle soreness. We will assist patients in dealing with these reactions through post—exercise guidance.
Outcomes {12}Primary outcome
Exercise level
Recorded using a brand of sports bracelet, referring to the International Physical Activity Questionnaire (IPAQ), which was developed by the International Physical Activity Measurement Group in 2001. Different types of exercise were assigned a value from 1 to 8 according to their metabolic equivalent. The level of exercise is calculated according to the formula “exercise level = metabolic equivalent × duration of each time × frequency” over the past seven days. The results are categorized into: (i) vigorous activity; (ii) moderate activity; and (iii) low activity. The Chinese version was introduced by Qu Ning et al. in 2004, with test–retest reliability ranging from 0.689 to 0.934 and criterion validity, using accelerometers, of 0.50.
Secondary outcome
Bath Ankylosing Spondylitis Functional Index (BASFI)
As a widely used international index, BASFI evaluates the overall physical function in AS through ten questions. The total score is the sum of individual question scores, ranging from 0 to 10, with higher scores indicating poorer function.
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
Another index assesses disease activity in AS and comprises six questions evaluating the patient’s symptoms over the past week. The total score is the average of the individual scores, ranging from 0 to 10, with higher scores indicating greater disease activity.
Fatigue Severity Scale (FSS)
This scale assesses the impact of fatigue on patients with AS. It includes nine items, and the FSS score is calculated as the total score divided by nine. Scores greater than 4 indicate severe fatigue, while scores of 4 or below suggest no fatigue or mild fatigue.
Quality of Life
Assessed using the Ankylosing Spondylitis Quality of Life (ASQoL) questionnaire, which consists of 18 questions. Each question is answered with a “yes” (scored 1) or “no” (scored 0). The total score is the sum of all item scores, with higher scores indicating poorer quality of life.
Exercise Adherence
This measure includes exercise completion rate and exercise compliance. Patients record their exercise behavior in a exercise diary. Exercise completion rate is calculated as the number of activities completed according to standard divided by the number of required activities. Exercise compliance is self-evaluated by patients against the standard for each activity, classified into: fully achieved (100% standard completion), mostly achieved (over 50% standard completion), occasionally achieved (below 50% standard completion), and not achieved (unable to meet the standard).
Participant timeline {13}
The study period spans from June 2025 to October 2026. The detailed timeline is presented in Table 2.
Table 2 Schedule of data collectionSample size {14}
To estimate the sample size using the BASFI as the indicator, the formula for sample size calculation based on the comparison of two sample means was applied.
$$n=2{\left[\frac{\left({u}_{a}+{u}_{\beta }\right)\sigma }{\delta }\right]}^{2}$$
With a significance level (α) of 0.05, power (β) of 0.1, and a two-sided test for differences, the standard deviation (σ) was set at 7.5 and the effect size (δ) at 5.375 based on similar experimental results [29]. Considering previous research experience, a preliminary dropout rate of 20% was estimated. Therefore, it was determined that each group would require 60 participants, resulting in a total recruitment of 120 individuals.
Recruitment {15}
The research subjects for this study are AS patients from hospitals of Guangdong Rheumatology and Immunology Specialty Alliance, which adopted a unified and standardized diagnosis and treatment planning for AS.