Paralleling the global surge in atrial fibrillation incidence [1]. Currently, both the prevalence of HBMI and the HAB have increased dramatically. The management of HBMI and obesity-associated cardiovascular diseases has thus become an increasingly significant challenge. The correlation between HBMI and AF/AFL burden may stem from shared risk factors, pathological mechanisms, and comorbidities linking obesity and AF. Specifically, obese patients exhibit excessive adipose tissue, which secretes adipokines, activates the renin-angiotensin system, and enhances oxidative stress. These processes trigger inflammatory responses, promote the migration and proliferation of smooth muscle cells, and impair endothelial function, thereby promoting the onset of cardiovascular diseases such as AF and CAD. These conditions mutually reinforce each other, forming a vicious cycle that collectively accelerates heart failure, stroke, and cardiovascular death, thereby increasing the burden associated with AF/AFL [2]. Furthermore, obesity-related expansion of epicardial adipose tissue is considered a key driver of AF, mediated through paracrine signaling and direct tissue infiltration [3]. A prospective cohort study on AF demonstrated that overweight and obesity were associated with an increased risk of major adverse events and mortality in AF patients [4]. However, some studies have reported a contradictory phenomenon—termed the “obesity paradox”—in which overweight and obese AF patients exhibit a reduced risk of cardiovascular and all-cause mortality [5]. A possible explanation is that overweight and obese patients typically receive earlier, more intensive treatment and closer follow-up monitoring, while obesity itself may provide greater metabolic reserve. Currently, it is of critical importance to comprehensively elucidate the HAB, including its sex- and region-specific disparities.
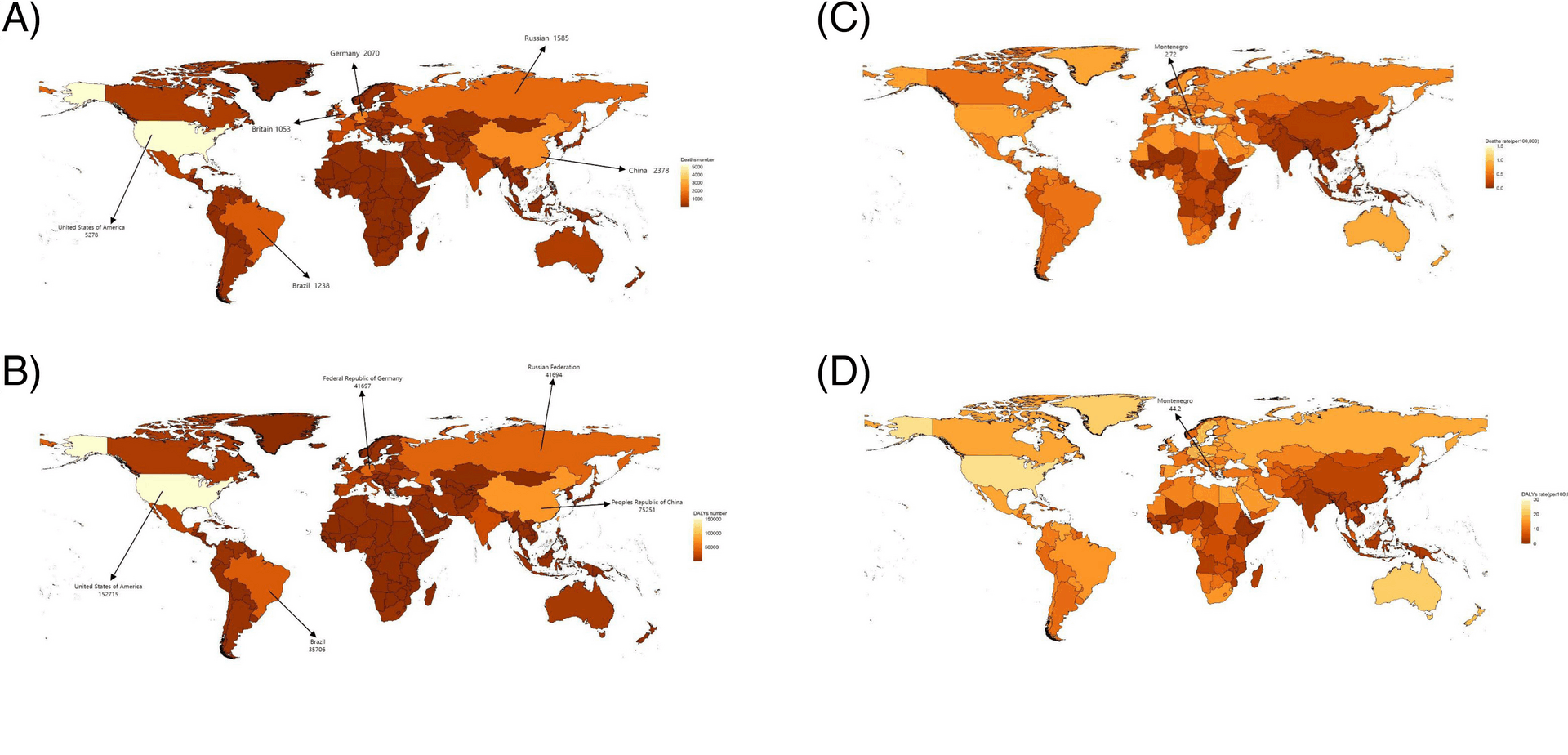
This study provides a comprehensive assessment of the HAB. The findings reveal that over the past three decades, HBMI-related AF/AFL has contributed to a significant increase in absolute deaths, DALYs, and age-standardized mortality and morbidity rates worldwide, with notable heterogeneity across regions, sexes, and temporal trends.
Across all SDI regions, ASDRs and ASMRs demonstrated consistent increases. Notably, the EAPC of ASDRs and ASMRs showed an inverse relationship with SDI (Supplementary Fig. 3 C), while absolute ASMRs and ASDRs values exhibited a positive correlation with SDI (Supplementary Fig. 1). This trend remained consistent when analyzed across bifurcated age groups (Supplementary Fig. 5). The HAB was highest in high-SDI regions, which accounted for 45% of the global AF/AFL burden in 2019 – representing a decline from 1990 levels yet still substantially exceeding the proportions observed in low- and middle-SDI regions. These findings align with the conclusions reported by Tan et al [6]. These observed patterns may be explained by the following factors: First, obesity-driven HBMI affects disease burden across all age groups as a prevalent metabolic disorder [7]. Second, the rapidly increasing obesity prevalence in low- and middle-SDI countries has accelerated the growth rate of HAB, thereby narrowing the inter-SDI disparity over time. Third, populations in high-SDI regions face greater obesity risks due to socioeconomic advantages that paradoxically facilitate unhealthy lifestyles—including tobacco use, alcohol consumption, and high-fat diets. This has led to particularly severe (and still worsening) obesity epidemics in developed nations, as exemplified by the extraordinary obesity rates documented in the United States [9]. Furthermore, existing studies indicate that elevated BMI is associated with an increased risk of cardiovascular disease events [8]. Thereby contributing to a greater burden of AF/AFL.
Notably, regions including East Asia, High-income Asia Pacific, South Asia, and Southern Latin America—despite their high economic development—exhibited lower mortality and disability rates without demonstrating the expected SDI-correlated disease burden and growth trends (Fig. 3C, Supplementary Fig. 1). This phenomenon may be attributed to ethnic, dietary, cultural, educational, and healthcare resource factors. For instance: Stronger weight-management motivation among Japanese women due to prevailing aesthetic standards [10]. Predilection for fish, shellfish, and plant-based diets in Japan and Korea-a dietary pattern significantly associated with reduced all-cause and cardiovascular mortality risks [11]. Lau et al. reported a higher prevalence of atrial fibrillation in Caucasian populations compared to Asia–Pacific nations such as China and Japan [12]. Although Australia, as part of the high-income Asia–Pacific region, exhibited elevated mortality and disability rates, its relatively small population size minimized its impact on aggregate regional data [13]. These region-specific determinants are not readily translatable to other settings for effective disease burden management. The pathogenesis of obesity involves complex multifactorial mechanisms, with economic development demonstrating a strong epidemiological association with obesity prevalence [14]. As economic growth continues to drive the obesity epidemic, regions with currently moderate SDI indices may inevitably experience disease burdens comparable to high-SDI regions without implementing comprehensive preventive strategies. Regrettably, current evidence indicates that despite widespread implementation of national weight-control interventions, no country has achieved sustained reversal of obesity trends, with the exception of temporary improvements observed during economic recessions.
Existing studies have demonstrated that the Gini coefficient serves not only as a measure of regional income inequality but also as a valuable tool for comparing disparities in disease burden across regions [15]. Our analysis reveals a consistent decline in the Gini coefficient for global HAB from 1990 to 2021 (Supplementary Fig. 8), suggesting progressive improvement in health inequality among nations. However, given the methodological constraints inherent in Gini coefficient calculations, the precise magnitude of this health inequality reduction remains undetermined.
Regarding gender disparities, to mitigate potential confounding by cultural, regional, and economic factors, we conducted stratified analyses by region and SDI level when comparing mortality and disability rates between sexes. The results consistently demonstrated significantly higher rates in women across nearly all subgroups (Supplementary Figs. 9A-9B). Similarly, the HAB was markedly greater in women—a pattern readily explained by their longer life expectancy (Supplementary Figs. 9C-9D) [16]. Current evidence further indicates that HBMI exerts a greater impact on AF disease burden in women than in men [17, 18]. This disparity may be attributed to both higher prevalence and greater severity of obesity among females [7]. In 2021, the prevalence of obesity among women was 20.8%, significantly higher than the 14.8% observed in men. Furthermore, Blüher et al. reported that severe obesity (BMI > 40 kg/m2) was 2.5 times more prevalent in women (1.6%) compared to men (0.64%) [19]. Furthermore, influenced by sex hormones and gut microbiota diversity, body composition exhibits significant heterogeneity between genders. At comparable BMI levels, women typically demonstrate lower muscle mass proportion and greater propensity for visceral adipose tissue accumulation [20, 21]. Women exhibit a higher prevalence of sarcopenic obesity and demonstrate greater susceptibility to the adverse cardiac functional impacts of central obesity compared to men [22]. Mechanistically, these effects may be mediated through sympathetic nervous system activation, pro-inflammatory responses, mitochondrial dysfunction, oxidative stress generation, and dysregulation of the renin–angiotensin–aldosterone system (RAAS). These pathways collectively contribute to vascular endothelial dysfunction, cardiomyocyte injury, and myocardial fibrosis [23, 24]. Potentially explaining why HBMI contributes disproportionately to cardiovascular disease incidence and AF/AFL burden in women.
A unique gender disparity pattern was observed in high-SDI regions, where male disability rates have consistently exceeded female rates since 1990—a trend contrasting with both other regions and mortality patterns within high-SDI areas (Fig. 2B). Age-stratified analysis (Supplementary Fig. 4) revealed an anomalous progressive trend: the age threshold at which male disability rates surpassed female rates shifted from 70–74 years (1990–1999) to 75–79 years (2000–2009), and further to 80–84 years (2010–2021). This phenomenon may be attributed to two synergistic factors: male AF patients demonstrate lower mortality risk [25], and obesity prevalence in high-SDI regions has undergone a gender crossover, with male rates now exceeding female rates [7].
The risk factor analysis revealed a substantial increase in the proportion of HAB among males since 1990, with the attributable fraction becoming significantly higher than in females (Supplementary Fig. 7). These findings suggest that over the past three decades, certain sex-specific factors have amplified the detrimental impact of elevated BMI more prominently in males compared to females. The accelerated progression in both prevalence and severity of obesity among male populations may represent a key explanatory factor for these observed epidemiological shifts [19].
AF/AFL is an age-related disorder, and the GBD data demonstrate that the AF/AFL burden attributable to obesity is disproportionately greater in older populations—a finding consistent with established pathophysiological mechanisms (Supplementary Figure S6B). Concurrently, current epidemiological data reveal a marked increase in obesity prevalence among young adults over recent decades [25]. This trend suggests that the full impact of contemporary obesity rates on future disease burden has not yet been realized. From a preventive perspective, targeted interventions including enhanced screening protocols and health education campaigns for this demographic should be prioritized to mitigate the anticipated surge in HAB.
Furthermore, it is noteworthy that AF induced by HBMI is not an isolated health issue. HBMI serves as a significant pathogenic factor for both AF and coronary artery disease (CAD) by inducing inflammation, oxidative stress, and endothelial dysfunction [26]. Simultaneously, CAD, being a major complication of AF, exhibits an intricate link with AF, forming a vicious cycle where each disease promotes the other.
Epidemiologically, extensive studies confirm that individuals with either AF or CAD are more susceptible to developing the other condition. Specifically, 18–40% of patients diagnosed with AF also have CAD [27]. At the molecular level, inflammation, oxidative stress, and endothelial dysfunction represent shared pathogenic mechanisms. AF contributes to endothelial dysfunction, which itself is an early manifestation of atherosclerosis [28]. AF promotes CAD by inducing endothelial dysfunction. Furthermore, CAD, often presenting as myocardial ischemia, exacerbates all three mechanisms underlying the initiation and perpetuation of AF: focal ectopic firing, re-entry, and neural remodeling [29]. Given that heart disease resulting from CAD is a leading cause of death globally, imposing a substantial healthcare burden and severely impairing public health [30], these findings underscore that controlling the epidemic trend of HBMI-induced AF offers substantial benefits that necessitates urgent intervention.
Study limitations
The potential influence of economic, healthcare, and cultural factors on GBD data sources remains incompletely quantified. While our use of SDI-stratified gender comparisons has substantially minimized potential confounding, residual biases may persist due to unmeasured regional variations in disease ascertainment and reporting practices. Second, Detailed information on different AF/AFL subtypes (such as paroxysmal, persistent, or permanent) cannot be obtained from the GBD database, which may limit the comprehensive and stratified analysis of the HAB. Third, similar to the classification of atrial fibrillation subtypes, the current analysis did not further stratify HBMI categories. Given variations in obesity prevalence and severity across nations and regions, the included populations may demonstrate significant heterogeneity between different geographical areas. While BMI remains a convenient and widely used screening tool for overweight and obesity, this metric fails to differentiate between fat mass distribution patterns. Consequently, BMI proves inadequate for characterizing geriatric obesity, abdominal adiposity, and visceral fat accumulation – all of which exhibit distinct metabolic consequences. Fourth,notably, GBD estimates are derived from modeled data rather than direct clinical measurements, potentially limiting their accuracy in certain national and regional contexts, particularly in settings with constrained surveillance infrastructur,furthermore, it is not possible to infer direct causality between HBMI and AF/AFL from these trend data.Future real-world studies are warranted to more precisely characterize disease trends and burden, particularly in low- and middle-SDI regions where data representativeness may be compromised. Fifth,