Lactate administration and sepsis
Sepsis is a major cause of mortality worldwide [52]. Hyperlactatemia is recognized as a marker of poor prognosis during sepsis [53], with its persistence despite optimal initial management (including fluid resuscitation and vasopressor use) participating in the definition of septic shock [54]. Increased anaerobic metabolism with tissue hypoxia and mitochondrial dysfunction were used to be considered as the primary mechanisms underlying hyperlactatemia in this condition [55]. However, in septic shock, hyperlactatemia is increasingly attributed to a hypermetabolic state combining sympatho-adrenergic-driven glycolysis and inflammation-related PDH inhibition [56]. In support of this, higher concentrations of lactate and pyruvate have been observed in skeletal muscle than in blood, suggesting increased local production in peripheral tissues [41].
To gain further insight, both experimental and clinical studies have explored the therapeutic potential of lactate in sepsis.
Lactate administration in experimental models
Several experimental studies have highlighted a potential beneficial effect of lactate in sepsis:
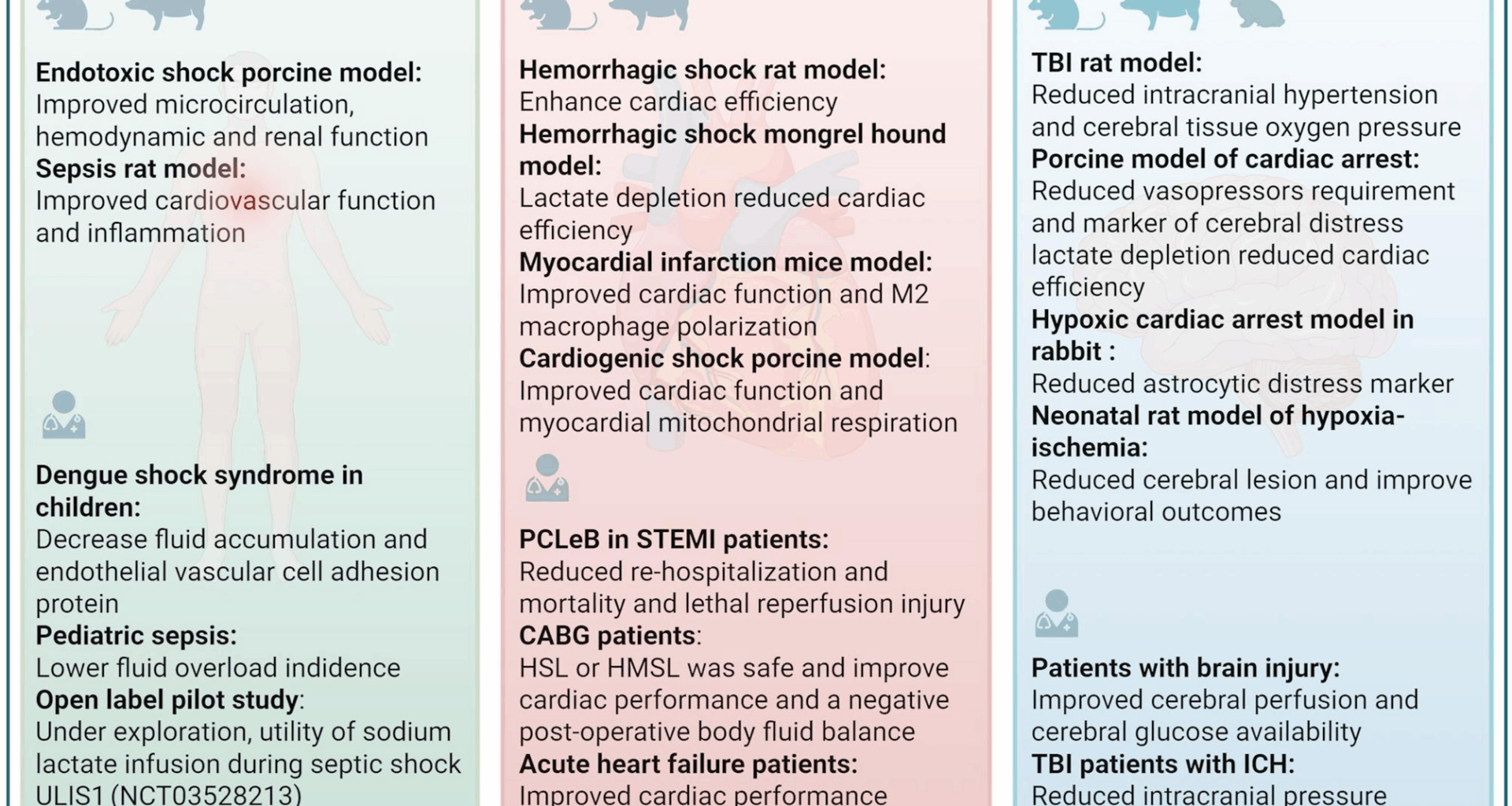
In an lipopolysaccharide-induced porcine endotoxemia model, HSL infusion (5 mL/kg/h) significantly improved mean arterial pressure and cardiac index in a two-fold manner in comparison with 0.9% NaCl, with even an improvement in microvascular reactivity and fluid balance, highlighting the ability of HSL to restore hemodynamics in this model, with effects on both macro and micro-circulation. Moreover, HSL administration was superior to a hypertonic control, consisting of 8.4% sodium bicarbonates (HSB), suggesting the beneficial effect was beyond the solely osmotic effect of the fluid. [57].
To further investigate the mechanisms of HSL, the authors repeated the protocol but included caloric supplementation (glucose) in the Normal saline (NS) and SHB groups, achieving a quantitative equivalent energetic input with HSL, and modified their protocol to target a mean arterial pressure > 65 mmHg (51). The HSL group had significantly better outcomes, including a lower fluid balance, higher microcirculation as measured by oxygen delivery (DO2) and lower plasma content of inflammatory cytokines. The authors concluded that HSL exhibited a metabolic and anti-inflammatory effects rather than merely an energetic or osmotic effect. In an ancillary study [58], histological analysis from the same experimental setup revealed that the HSL group had significantly fewer thrombosed glomerular capillaries and lower thrombin-antithrombin complexes. Furthermore, platelet count and fibrinogen levels deteriorated earlier and more significantly in the control groups than in the HSL group [58]. Therefore, this model suggests the use of HSL during acute and severe inflammation as potentially beneficial.
But endotoxemia is no sepsis. In this objective, our team developed a rat model of cecal ligation and puncture-induced sepsis adapted to fluid infusion, using a dedicated jugular catheter. Continuous infusion of HSL, SHB or NS (both supplemented with glucose) at 2.5 mL/kg/h for 18 h presented drastic different results. HSL administration significantly improved mesenteric microcirculation, cardiac output, and fractional shortening compared to NS group. Pro-inflammatory cytokines were also significantly lower in the HSL group compared to NS group. HSL also led to a more negative fluid balance and reduced capillary leakage (measured via Evans blue dye) in multiple organs. Therefore, in this model, HSL was also beneficial to sepsis.
All these experimental studies demonstrated improvements in macro- and microcirculatory parameters during acute inflammation. However, the underlying mechanisms remain incompletely understood, as lactate exerts its effects through both metabolic and cell signaling pathways. On one hand, lactate may contribute to metabolic adaptation during energy failure, serving as a substrate for oxidation or conversion into fatty acids. On the other hand, it may modulate inflammatory responses via activation of the GPR81 receptor and suppression of inflammasome activity. This latter mechanism has been investigated in a rodent model of acute pancreatitis, where the use of small interfering RNA targeting GPR81 confirmed the receptors involvement in inflammation associated with this condition [59].
While pancreatitis and sepsis involve distinct inflammatory pathways, both share key features of systemic inflammatory response and metabolic reprogramming. Therefore, although caution is warranted, it remains plausible that GPR81 may also play a regulatory role in the inflammatory processes of sepsis, a hypothesis that warrants further investigation.
Furthermore, in experiments conducted by our team, lactate infusion was associated with elevated levels of the ketone bodies β-hydroxybutyrate and acetoacetate, which serve as critical energy substrates for the myocardium. These findings suggest that the conversion of lactate into acetyl-CoA—potentially via lactyl-CoA synthase—may represent a key metabolic pathway supporting energy production during inflammatory states.
In contrast to previous studies reporting beneficial effects of lactate, one investigation demonstrated potentially harmful outcomes in sepsis. In a hyperdynamic septic shock model induced by fecal peritonitis in sheep [60], administration of half-molar sodium lactate (HMSL)—given as a 3 mL/kg bolus over 15 min followed by continuous infusion at 1 mL/kg/h at 2, 6, and 10 h post-sepsis induction—was associated with significantly poorer outcomes compared to hypertonic saline (3% NaCl) and RL. Specifically, HMSL led to reduced mesenteric perfusion (196 ± 55 mL/min vs. 310 ± 61 for NaCl and 109 ± 25 for RL), greater capillary leakage as indicated by a lower PaO₂/FiO₂ ratio (211 ± 45 mmHg vs. 368 ± 38 and 313 ± 46), and a lower mean arterial pressure (67 ± 7 mmHg vs. 89 ± 6 and 78 ± 11) at 12 h. Median survival was also reduced (17 h for HMSL vs. 22 h for HSL and 20 h for RL). The discrepancy with previous studies may be explained by several key experimental differences. In this study, fluid resuscitation was initiated with a significant delay—two hours after the onset of septic shock—which may have compromised the hemodynamic response. Additionally, the resuscitation strategy aimed to maintain pulmonary artery occlusion pressure rather than mean arterial pressure, possibly resulting in suboptimal tissue perfusion. The administered lactate dose was also considerably lower than in prior studies, approximately half the dose used in those experiments. This suboptimal resuscitation strategy may have led to inadequate tissue perfusion, which could explain the absence of an increase in blood glucose and the progressive rise in the lactate/pyruvate ratio—findings indicative of impaired lactate metabolism, potentially due to lactate dehydrogenase inhibition or mitochondrial dysfunction. Moreover, the study involved a lethal model of septic shock, where the extent of organ and mitochondrial damage may have been too severe to allow any beneficial effect of lactate metabolism to emerge.
Lactate administration in clinical studies
Only a limited number of studies have investigated the use of hyperosmolar lactate-containing fluids in human sepsis, largely due to the overall lack of favorable data on hypertonic solutions and the still scarce evidence from experimental models.
A first single-blinded trial explored the use of HMSL in a pediatric population with dengue shock syndrome [61], with RL as comparator. The protocol used a large amount volume of fluids and the administration of hydroxyethyl starch (HES) as rescue in case of persistent circulatory failure. There was no difference in the total amount of HES administered between the two groups during the first 12 h. HMSL administration significantly reduced endothelial dysfunction at 24 h, as evidenced by lower circulating levels of Vascular cell adhesion protein 1 (VCAM-1). It was also associated with a significantly threefold lower net sodium-water balance over 24 h (35 ± 10 mL/kg vs 107 ± 7 mL/kg). No difference was observed in survival, but the trial was not designed for this purpose. Furthermore, lactate administration was well tolerated, with no differences observed in pH, osmolality, or sodium and lactate plasma levels between the groups.
Secondly, in an open-label, prospective, randomized study conducted in a pediatric population with sepsis [62], the administration of HSL (a 5 mL/kg bolus followed by a continuous infusion of 1 mL/kg/h for 12 h) was compared to NS (20 mL/kg bolus followed by administration based on fluid requirements/kg body weight for 12 h) in addition to standard care (antibiotics administered within the first hour). HSL administration, compared to NS, resulted in greater lactate clearance at 1 and 6 h: 20.5 (−228.5–80.3) vs −15.5 (−185.7–44.4) and 33.3 (−71.4–90.9) vs −16.7 (−135.0–62.5), respectively. Additionally, fewer patients in the HSL group experienced fluid overload greater than 10% (0 vs. 15 patients). HSL administration was well tolerated with respect to serum sodium levels. There were no differences in survival rates or the time to resolution of septic shock between the two groups. This trial demonstrated that lactate infusion may help ameliorate metabolic dysfunction in the context of sepsis. Notably, despite exogenous lactate administration, plasma lactate levels may still serve as a surrogate marker for circulatory status. Clinicians may be concerned that administering lactate could interfere with its interpretability as a prognostic biomarker. However, these findings support the continued relevance of plasma lactate monitoring, although confirmation through additional studies is necessary. Importantly, we cannot exclude the possibility that lactate clearance varies depending on the severity of sepsis and the functional status of key organs—particularly the liver and kidneys, which are central to lactate metabolism.
Overall, hypertonic sodium lactate in experimental sepsis has demonstrated: (a) Metabolic effects through improvements in microcirculation, fluid balance, lactate clearance and cardiac function (cardiac output and shortening fraction); (b) Anti-inflammatory effects by reducing vascular thrombosis, decreasing the expression of pro-inflammatory cytokines, and lowering vascular cell adhesion molecule levels. It has also suggested improvement in human sepsis with reduction in fluid overload and metabolic status, despite evidence is far too spare to recommend its routine utilization for the moment. An ongoing French open-label pilot study is currently evaluating the utility of molar sodium lactate infusion in the management of septic shock (ULIS1, NCT03528213). The primary outcome measure for this study will be fluid balance at 48 h post-initiation of treatment and safety and other outcomes of interest will also be explored.
Lactate administration in acute heart failure
Myocardial infarction (MI) is a leading cause of morbidity and mortality. [63]. Its treatment involves blood flow restoration and ongoing management to prevent further events. During ischemia, reduced oxygen supply impairs aerobic respiration, leading to an increase in anaerobic glycolysis and subsequent lactate production. Paradoxically, the reintroduction of oxygen can exacerbate injury through the generation of reactive oxygen species and calcium overload, a phenomenon known as reperfusion injury [64, 65] with an ATP depletion. Under physiological conditions, fatty acids represent the primary energy substrate for the myocardium [66]. Lactate accumulation has been shown to promote fatty acid synthesis over oxidation, through activation of acetyl-CoA carboxylase [42]. While this may reflect a shift in metabolic preference, the extent to which reduced fatty acid oxidation directly explains lactate’s role as an alternative energy substrate during ischemic episodes remains debated [67, 68]. Lactate has also been implicated in the regulation of cellular redox balance. [69].
In the context of acute heart failure, recent scientific investigations have been initiated to explore the potential advantages of targeted lactate modulation as a strategy to optimize myocardial energetics and alleviate the repercussions of impaired cardiac function.
Lactate administration in experimental models
Perfusion of isolated hearts with lactate (8 mM), glucose (11 mM), and palmitic acid at physiological concentrations (0.4 mM) was associated with a statistically significant increase in cardiac work and cardiac efficiency (cardiac work/oxygen consumption) over a period of one hour compared to perfusion with glucose and/or palmitic acid alone in rats subjected to hemorrhagic shock [70]. Furthermore, experiments utilizing dichloroacetate, an antagonist of metabolic action of lactate, in mongrel canine, have shown that deprivation of myocardial lactate during resuscitation from hemorrhagic shock decreased stroke volume and myocardial efficiency [71]. These data argue, for a direct beneficial effect of lactate molecule on myocardial systolic function. Moreover, its administration during experimental sepsis improved both systolic and diastolic function [50].
Lactate infused in mice with definitive myocardial infarction showed cardioprotective proprieties, as mice presented enhanced cardiac performance, elevated proportion of anti-inflammatory macrophages, reduced cardiac myocytes apoptosis, and increased neovascularization [72]. Altogether, experimental studies suggest that lactate administration may have beneficial effects on both systolic and diastolic function across various heart failure models, as well as cardioprotective properties, highlighting its potential for clinical human trials.
Finally, in a porcine model of cardiogenic shock induced by occlusion of the left main coronary artery [73], the administration of HSL, compared to hypertonic sodium chloride, was associated with a significant increase in cardiac output without concomitant increases in heart rate or arterial blood pressure. Notably, lactate infusion improved ventriculo-arterial coupling efficiency, with further enhancement observed when combined with dobutamine. Urine output and central venous oxygen saturation were also significantly elevated in the HSL group. At the molecular level, mitochondrial complex I activity—responsible for NADH oxidation and electron transfer to ubiquinone—was significantly increased in animals receiving HSL.
Lactate administration in clinical studies
Again, very few studies are available in humans. In a cohort comprising 100 patients, the administration of RL solution during coronary reperfusion within 12 h of the onset of ST-segment elevation myocardial infarction (STEMI) resulted in no in-hospital or one-year mortality, as well as no re-hospitalization for heart failure during the one-year follow-up [74]. However, the absence of a control group limits the interpretation of these results. This strategy, called postconditioning with lactate-enriched blood (PCLeB), has been investigated in small-scale cohort studies and appears to be effective in mitigating reperfusion arrhythmia, myocardial stunning, microvascular reperfusion injury, and lethal reperfusion injury in STEMI patients [75]. Additionally, PCLeB has been associated with a lower increase in C-reactive protein levels in STEMI patients, confirming anti-inflammatory proprieties of lactate, and favorable short- and long-term outcomes, including reduced plasma NT-pro brain natriuretic peptide levels [75]. In light of the experimental studies cited above, lactate administration may offer myocardial protective properties in the context of ischemia, which warrants further exploration. This also raises the question of whether higher doses of lactate, such as in molar or semi-molar solutions, could be beneficial. In a crossover study, the administration of HSL at a rate of 3 mL/kg over 20 min, followed by a 2 mL/kg infusion over 60 min, was safe in healthy subjects. Compared to 3% hypertonic saline perfusion, HSL was associated with a significant increase in cardiac output (CO) and left ventricular ejection fraction by 0.51 L/min and 4%, respectively. The pH, bicarbonate and simplified strong ion difference (SID) significantly increased while potassium levels significantly decreased during HSL perfusion [76]. Similarly, 14 to 16 h after an elective coronary artery bypass grafting (CABG), the infusion of HSL solution in patients with and without extracorporeal circulation was associated with a small but significant increase in blood glucose levels (8% and 17% from basal respectively) and oxygen transport. However, oxygen extraction was significantly lower in patients receiving HSL compared to those receiving hypertonic saline [76, 77], questioning the place of these fluids in this setting. Additionally, an open randomized study compared the infusion of HMSL and 6% HES at a dose of 3 mL/kg within 15 min at the beginning of CABG surgery. HMSL infusion was associated with a slightly but significant increase in heart rate (mean 6.86 ± 20.34 beats per minute), an increase in CO (mean 0.5 ± 1.02 L/min/m2), and a small yet significant decrease in the systemic vascular resistance index [78]. At the end of surgery, the fluid balance of patients who received HMSL was significantly negative at − 445.94 ± 815.30 mL, compared to patients who received HES, who exhibited a positive fluid balance of 108.48 ± 1219.90 mL. Moreover, patients who received HMSL had a significant increase in pH, base excess, serum sodium, and lactate levels, along with a significant decrease in serum chloride levels, confirming the effective assimilation of these fluids. Similarly, a pilot randomized, open-label controlled study in patients with acute heart failure compared the administration of HMSL over 24 h to a single infusion of RL [79]. After administration, patients who received HMSL showed a significant improvement in cardiac function, with an increase in CO of 1.44 ± 2.34 L/min at twenty-four hours and 0.82 ± 2.75 L/min at forty-eight hours. Patients who received RL, on the other hand, did not exhibit a significant increase in CO or tricuspid annular plane systolic excursion (TAPSE). The fluid balance of patients who received HMSL was negative by − 843 mL at 24 h and − 1.909 mL at 48 h compared to 586 mL at 24 h and − 598 mL at 48 h in the RL’s patients. There was no difference in terms of vasopressor drugs between the two groups. HMSL administration led to a significant rise in plasma sodium (from 136 ± 4 to 146 ± 6 mmol/L) and pH (from 7.40 ± 0.06 to 7.53 ± 0.03), while levels of potassium, chloride, and phosphate were reduced.
In summary, sodium lactate at molar or semi-molar concentrations in acute heart failure has demonstrated (a) an osmolar effect by reducing the input–output balance and modifying pH compared to solutions with a lower osmotic potential; (b) an energetic effect by improving parameters of left and right systolic function (CO, ejection function, TAPSE) and diastolic function, likely through increased oxygen transport and elevated production of 3-hydroxybutyrate during lactate perfusion [50]. Indeed, its perfusion has been associated with enhanced cardiac function [80]; (c) anti-inflammatory effects by lowering the proportion of anti-inflammatory macrophages, reducing cardiac myocyte apoptosis, and increasing neovascularization. Therefore, lactate infusion may be a potential tool to improve outcomes of patients presenting acute cardiac injuries, but further trials are mandatory to identify patients who most benefit from this therapy, among surgical, post- or-per-MI or cardiogenic shock.
Lactate in brain injury
Lactate is a preferred metabolite for the brain during rest, exertion, as well as in situations of profound hypoglycaemia or acute brain injuries [33,34,35]. Thus, its deprivation or supplementation may modify cell prognosis during injury. Brain injury (BI) can arise from traumatic (TBI) or non-traumatic causes (NTBI). BI manifests as two types of lesions: primary lesions, typically irreversible, and secondary lesions, which may be amenable to control and reduction through therapeutic interventions [81]. During BI, there is a shift in brain glucose metabolism, characterized by an elevation in lactate production and consumption [82,83,84]. In patients with TBI, systemic lactate production was shown to increase by 71% compared to healthy controls, while cerebral glucose utilization was suppressed. Notably, up to 67.1% of newly produced glucose was derived from lactate via gluconeogenesis, reinforcing the concept of lactate as a key substrate supporting brain metabolism during injury [85]. Current understanding suggests that astrocytes play a pivotal role in mediating lactate production during BI through two primary mechanisms known as “hyperglycolysis” (production via glutamine signaling) and “glycogenolysis” (the breakdown of glycogen stored in astrocytes) [86]. Recent studies have explored the therapeutic potential of lactate in the context of BI.
Lactate administration in experimental models
In a rat model of TBI, the administration of HMSL (1.5 ml/kg body weight on the first hour following the trauma) significantly reduced intracranial hypertension (ICH) from 24 to 12 mmHg compared to the control group (1.5 ml/kg body weight of isotonic saline solution on the first hour following the trauma). However, this decrease in ICH was not significantly different from that induced by mannitol perfusion (1.5 ml/kg body weight of 20% mannitol on the first hour following the trauma). Similarly, HMSL perfusion was associated with an increase in brain tissue oxygen tension (PtiO2) of 19 ± 8.66 mmHg compared to controls. However, this increase was not significantly different from the PtiO2 increase observed in the mannitol group, which rose by 10 ± 4.6 mmHg. The authors attributed this effect to the increased expression of aquaporin 4 (facilitating extracellular water transport) and the K⁺–Cl⁻ Cotransporter 2 (KCC2)-enabling chloride and potassium efflux accompanied by water, observed at 24 h and from 4 h post-HMSL administration, respectively, despite the link with lactate infusion is far from evident [87]. Importantly, this effect lasted for a more extended period and occurred without an increase in osmolality, in contrast to mannitol, suggesting a mode of action likely to be metabolic rather than solely osmotic [87].
In a porcine model of cardiac arrest (CA) induced by ventricular fibrillation, administering HMSL during the arrest (bolus of 10 mmoL at CPR initiation followed by a continuous infusion of 30 μmol/Kg/min for 12 h after resuscitation) or after the restoration of cardiac rhythm (bolus 20 mL of NaCl 0.9% at the beginning of CPR followed by a continuous infusion of 30 μmol/Kg/min of HMSL for 12 h after resuscitation) resulted in lower circulating levels of glial fibrillary acid protein (marker of cerebral distress), and significantly fewer instances of a flat electroencephalogram trace 12 h after resuscitation (63.6% vs 100%) compared to the control group (Bolus of 20 mL of NaCl 0.9% at the beginning of CPR manoeuvres and a continuous infusion of balanced crystalloids for 12 h after resuscitation) [88]. However, in this study, there was no difference in intracranial pressure or PTiO2 between the two groups and the control group. These findings align with those from a study on a hypoxic CA model in rabbits (12.5 min of untreated CA), where HMSL administration (5 mL/kg/hr for the 120 min) after the restoration of cardiac rhythm led to improved pupillary reactivity and lower circulating levels of S100β (an astrocytic distress marker) compared to the control group (5 mL/kg/hr of NaCl 0.9% for 120 min): 83 vs 25% and 189 ± 42 pg/mL vs 412 ± 63 pg/mL respectively [89]. Experimental data from a neonatal rat model of hypoxia–ischemia (HI) demonstrated that lactate reduced the volume of cerebral lesions and improved behavioral disturbances remote from the HI event [90]. In the case of the three studies mentioned above, the lactate effect is likely secondary to the energetic contribution of lactate, which limits neuronal apoptosis and then improve neuronal function. Furthermore, lactate supplementation may contribute to preventing post-stroke neurodegeneration [91].
Lactate administration in clinical studies
In a prospective interventional study involving patients with acquired BI, HSL administration for 3 h, during the 39 h [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] following BI, improved cerebral perfusion: + 36% [21–66%] increase in middle cerebral artery mean cerebral blood flow velocities and increased cerebral glucose availability: + 42% [30–78%] [92]. During HL administration, systemic sodium levels and plasma osmolality increased, yet remained within safety thresholds (≤ 155 mmol/L and ≤ 320 mOsmol/L, respectively) [92]. In another prospective interventional study involving patients with acquired BI, HSL administration for 3 h during the 72 h following BI was associated with a decreased of intracranial pressure: −0.86 [−1.47 to −0.24]; brain tissue oxygen tension reduction: −0.58 [−1.14 to −0.01] [93]. However, these studies lacked a control group.
In a randomized open-label study involving severe TBI patients with ICH, the administration of HMSL (1.5 ml/kg over 15 min) resulted in a more significant and prolonged reduction in intracranial pressure compared to mannitol administration (1.5 ml/kg of mannitol 20% over 15 min): −5.9 ± 1 vs −3.2 ± 0.9 mmHg at the fourth hour respectively. Plasma osmolality, serum sodium, and pH were significantly increased in patients receiving HMSL (15). A randomized study (NCT05004610) is currently underway with the hypothesis that the administration of HSL during cardiac arrest would reduce cerebral damage, with the assessed marker being the level of Neuron Specific Enolase.
These effects are due to (a) an osmolar effect, as evidenced by cerebral water loss during its administration, whereas the administration of RL in the context of brain trauma is deleterious [94]; and (b) a metabolic effect, demonstrated by the increased availability of cerebral glucose during its administration, the reduction in neuronal distress markers, and the sustained cerebral water loss over time without an associated increase in osmolarity. This effect may be mediated by ketone bodies, particularly 3-ß-hydroxybutyrate, which has been found to increase during HSL infusion (50). Its administration at a dose of 0.18 g/kg/h over 12 h in swine that underwent cardiac arrest due to electric shock was associated with a neuroprotective effect (significantly lower plasma biomarkers of brain injury, improved electroencephalographic recordings) compared to the infusion of a balanced solution [88].