Study design
This study was registered as a randomized controlled trial (RCT) under the number NCT05463588 on 08/07/2022 at ClinicalTrials.gov and was retrospectively registered. It was conducted and reported in accordance with the CONSORT guidelines.
ParticipantsInclusion criteria
Participants had no recent surgery (6 months), uncontrolled diseases, vertigo, joint/knee issues, or severe balance problems; Body Mass Index (BMI) < 40; and no regular pain medication use during the study.
Exclusion criteria
Excluded if uncontrolled diseases, vertigo, balance/joint disorders, recent surgery, cognitive issues, pregnancy, incomplete fall risk tests, missed follow-ups, or lack of consent.
Sample size calculation
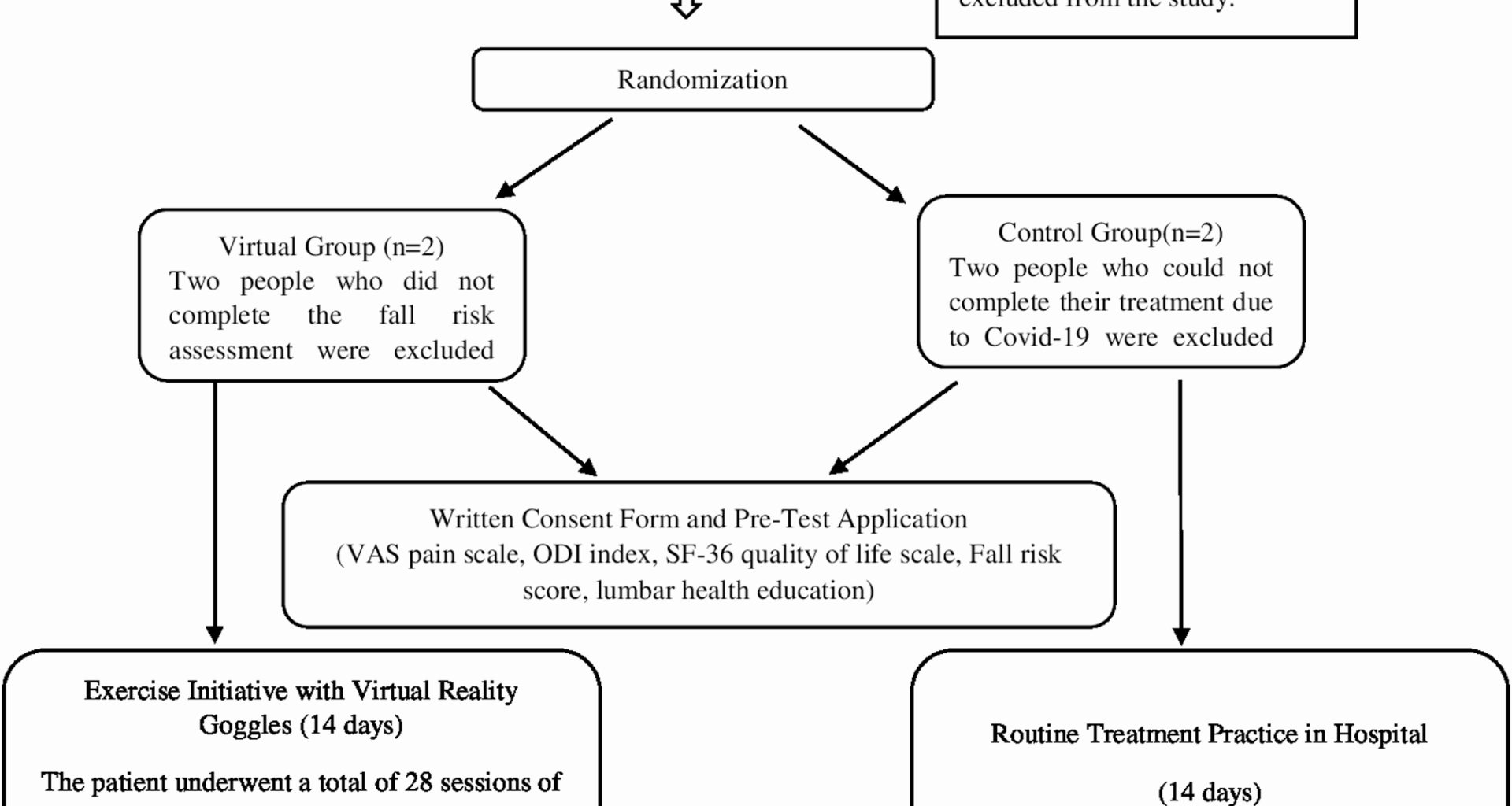
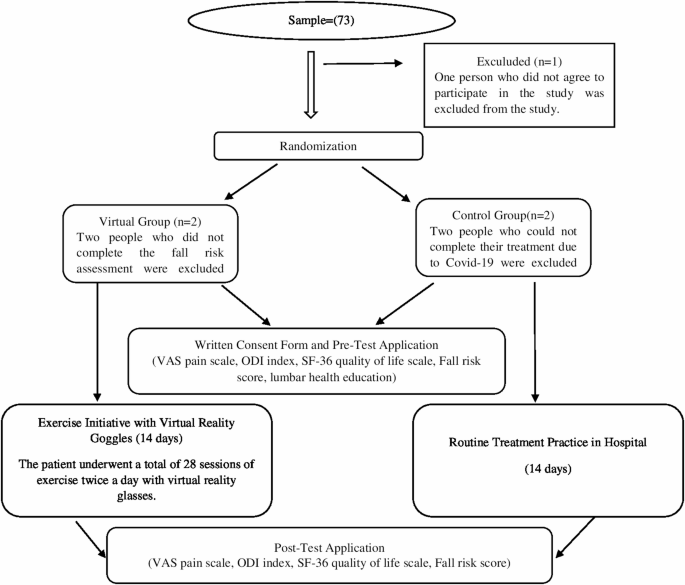
Between September 2021 and March 2022, 230 patients were treated at the Physical Therapy and Rehabilitation Unit of Atatürk University Hospital. An a priori power analysis indicated that 64 participants would be sufficient to detect significant effects [41]. During the study, 68 eligible patients who consented were enrolled. Diagnoses were confirmed by a senior physical medicine and rehabilitation specialist with over ten years of experience in musculoskeletal rehabilitation. Sample size was determined using G*Power (v3.1.9.7) based on a clinically meaningful difference in primary outcomes, with an effect size of 0.99, power of 0.80, and alpha of 0.05. No interim analyses or stopping guidelines were organized or implemented throughout the trial. Initially, 73 patients were considered; 5 were excluded (2 COVID-19, 1 declined, 2 incomplete fall risk data), leaving 68 participants (34 VR group, 34 control). Post-hoc analysis confirmed a large effect size (1.00) and power (0.98) at 95% CI. Randomization was done via MedCalc 14, with allocation concealed by opaque envelopes. Power analysis focused on comparing post-test VAS pain scores between groups.
Randomization and allocation concealment
Randomization was performed using MedCalc 14 software, generating a computer-based sequence to ensure unbiased group assignment. Participants had equal chances of being allocated to VR or control groups. An independent researcher, uninvolved in intervention or assessment, managed group assignments. Allocation concealment was maintained using opaque envelopes, keeping assignments confidential until intervention, preventing team bias.
Blinding inability
Blinding was not feasible due to the visible nature of VR intervention, so participants and researchers were aware of group allocation, posing potential bias. To mitigate this, rigorous randomization and allocation concealment were applied. ata were analyzed using Statistical Package for the Social Sciences (SPSS) version 20. Descriptive statistics and normality tests were performed. Independent and paired t-tests, Chi-square tests, effect sizes, and Cronbach’s alpha were used based on data type and research questions.
Control of confounding variables
Potential confounders (e.g., age, gender, BMI, baseline pain, functional scores) were controlled by strict inclusion/exclusion criteria. Homogeneity between groups was verified using t-tests and chi-square tests, showing no significant baseline differences (p > 0.05). Intention-to-treat analysis was applied. Missing data were handled using multiple imputation.
InterventionsVR group
“The VR group received standard physiotherapy modalities, including electrotherapy and heat application, alongside lumbar exercise training. Exercises demonstrated during the initial session were later performed using the VR headset. A video with 13 different’Back School Exercises’was shown through the headset, with each exercise repeated at least five times. The first exercise was selected based on the patient’s preference and condition. To ensure hygiene, hygienic head pads were used with the VR headset. The researcher was present to intervene in case of emergency.
Control group
The control group received standard physiotherapy modalities, including electrotherapy and heat application, along with lumbar exercise training aimed at improving flexibility, strength, and mobility of the lumbar spine. These exercises were performed in the exercise room during scheduled appointments, without the VR headset. After the study, interested control group patients were offered the opportunity to perform exercises using the VR headset.
Intervention detailsControl group
Participants in the control group received a single, 30-min educational session. This session was delivered through a PowerPoint presentation, direct instruction, and a question-and-answer format. The education covered the anatomy of the lower back, causes of lumbar disc herniation, preventive measures, and recommended lumbar exercises. Following the session, participants independently performed the learned exercises over a total of 28 sessions without the use of virtual reality glasses.
Study Group (SG)
Participants underwent VR-based exercise therapy twice daily for 28 sessions. Sessions started at ~ 25 min, reducing to ~ 20 min from week two as strength and flexibility improved. Exercise intensity increased progressively with gains in joint mobility, muscle strength, and movement familiarity. VR videos recorded with a 360° GoPro Max showed four physical therapists demonstrating exercises simultaneously, guided verbally by an instructor. Each exercise was repeated five times per session, with two sessions daily throughout the intervention.
Adherence monitoring
Patient adherence was tracked via daily attendance records and researcher supervision. Participants reported any exercise completion barriers. Adherence rate was calculated as the percentage of completed sessions out of the planned 28.
Number and duration of exercise practices
The standard physical therapy for LDH lasts 3 weeks (15 weekdays). With the first day reserved for lumbar education, the VR group (SG) had 14 days for exercise therapy, completing two sessions daily, totaling 28 sessions. Patients wore comfortable clothing, and sessions were paused if needed, resuming from the last completed movement.
Intervention environment and materials
Exercise Room: A 5 × 4 m2 room equipped with a mat and a VR headset for lumbar exercises. No other equipment was used; safety protocols were observed.
Lumbar health protection booklet
Prepared from back school programs, it details lumbar anatomy, posture, and protective exercises with text and illustrations. Reviewed by ten experts prior to use.
Virtual reality headset
Device specs included 64 GB storage, 2560 × 1440 resolution, and 470 g weight. Thirteen instructor-led lumbar exercise videos were preloaded.
Outcome measuresQuestionnaire
A 20-item questionnaire developed based on the literature [2, 27, 28].
Visual Analog Scale (VAS)
Originally developed by Hayes and Patterson in 1921 to assess pain intensity [43]. Participants rate their pain on a 10 cm scale, where 1–4 indicates mild pain, 5–6 moderate pain, and 7–10 severe pain [44]. In this study, the VAS was specifically used to assess low back pain intensity in individuals with lumbar disc herniation.
Oswestry Disability Index (ODI)
Created by Fairbank et al. in 1980 to assess disability from low back pain [45]. Scores are between 0–100%, with higher scores indicating more severe disability. The Cronbach’s α coefficient in this study was 0.92.
SF-36 quality of life scale
Developed by Ware in 1987 to assess the physical and mental health-related quality of life [46]. It consists of 36 questions across 8 sub-dimensions: Physical Function, Social Function, Physical Role Function, Emotional Role Function, Mental Health, Energy and Fatigue, Pain, and General Health. The scores range from 0–100, with higher scores indicating better health. The Cronbach’s α for this study ranged from 0.41 to 0.90. The Emotional Role Function sub-dimension was excluded due to low reliability.
Fall risk measurement
Calculated using the Tetrax FB posturography device (Model: Tetrax FB, Manufacturer: Sunlight Medical). The patient places their bare feet on designated pressure areas, and a computer program calculates the fall risk score. Scores are categorized as”Mild Fall Risk”(0–36%),”Medium Fall Risk”(36–58%), or”High Fall Risk”(58–100%) [47].
Variables of the studyIndependent variable
Exercise performed using VR headsets.
Dependent variables
Visual Analog Scale (VAS), Oswestry Disability Index (ODI), quality of life scores, and fall risk scores.
Data collection
Conducted via face-to-face interviews using standardized tools, gathering demographic, clinical, pain, medication, and adherence data.
Ethical principles
Approved by Atatürk University Ethics Committee; participants provided informed consent and were informed of withdrawal rights. Study adhered to the Declaration of Helsinki and was registered (NCT05463588). Participants completed questionnaires as part of data collection; nevertheless, patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research. No adverse events or harms were recorded or reported during the intervention period. Participant safety was observed closely by the research team during the study. No important changes were made to the trial protocol after the onset of the study.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 20. Descriptive statistics and normality tests were performed. Independent and paired t-tests, Chi-square tests, effect sizes, and Cronbach’s alpha were used based on data type and research questions. No additional analyses were completed.
CONSORT flow diagram
(Fig. 1 CONSORT diagram).