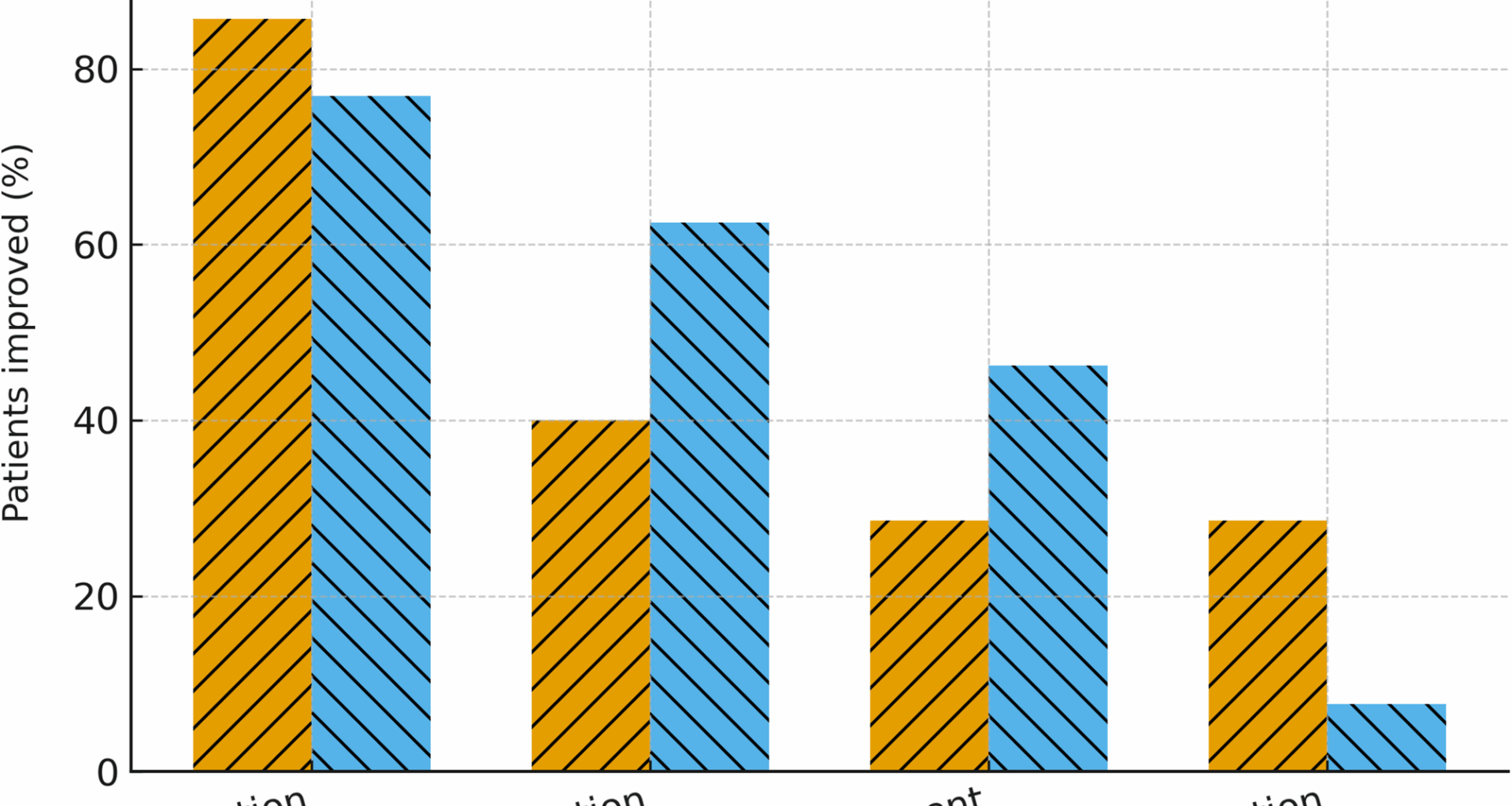This retrospective study evaluated the effects of CGs in adults with HSD or hEDS. We observed effectiveness (notably a pronounced reduction in pain) in 80% of the patients. These results are in line with the small amount of literature data on CG use in hEDS. Furthermore, the present study is one of the first to have included patients with HSD.
Clinical interpretation
Our observation of a reduction in pain is in line with Bénistan et al.’s22 report of significant decreases in (i) pain at the most affected joint, (ii) neuropathic components, and (iii) joint instability. Over half of our participants reported a reduction in analgesic use (excluding acetaminophen), which underscores the potential economic value of a CG-based approach. However, given our study’s retrospective design, we could not systematically assess the analgesic drug classes and initial dose levels or reductions in dose levels or dosing frequency. Although this limitation ruled out a detailed assessment of the total medication burden, the observed trend towards drug sparing might be clinically relevant and warrants confirmation in prospective studies.
An improvement in QoL was reported by only 40% of patients, and a reduction in fatigue was reported by 15%; these low values reflect the multifactorial nature of fatigue in this patient population (with sleep disturbances, deconditioning, etc.)22. CGs appear to be more effective for locomotor and proprioceptive symptoms but have only a limited systemic impact.
Similar treatment response rates in HSD vs. hEDS patient groups
There were no significant differences in the endpoints between patients with HSD and patients with hEDS. Although the proportion of patients with an alteration in visceral proprioception was higher in the hEDS group, both groups responded to CGs to a similar extent. This novel finding has not been previously reported in the literature and suggests that patients with HSD may benefit from CGs as much as those with hEDS do.
The comparison of our present results with previous studies is challenging because the effects of CGs on patients with HSD had not been specifically evaluated; previous studies focused exclusively on individuals with hEDS. Nevertheless, it is known that some aspects of the disease mechanism are common to HSD and hEDS and that the two disorders share a broad spectrum musculoskeletal and proprioceptive symptoms [1]. These results lend support to the concept of a common sensory–motor dysfunction that underlies the two ying both disorders and justify the future inclusion of HSD in prospective studies of compression therapy.
In France, reimbursement of CGs by the national health insurance fund currently covers the indication of hEDS but is frequently refused in an indication of HSD, owing to insufficient scientific evidence. Our studymight justify the reconsideration of reimbursement criteria and providing access to a broader patient population.
Predictors of the treatment response
The time per day wearing CGs was a strong predictor of a treatment response (p = 0.0013; r² = 0.45) and thus highlighted the importance of treatment adherence. In line with this finding, intolerance to CGs was significantly associated with the absence of clinical benefit (p = 0.001). These preliminary observations suggest that sustained use of CGs favors better clinical outcomes, although this hypothesis requires confirmation. The apparent lack of a statistically significant correlation between the Beighton score and clinical effectiveness confirmed that the severity of hypermobility alone is not predictive of a response to CGs; in fact, a documented impairment in proprioception might be more relevant.
Interestingly, a higher BMI was significantly associated with a more frequent clinical response to CGs. One plausible hypothesis is that in individuals with a greater body volume, CGs might achieve a more uniform pressure distribution across subcutaneous tissues and might thereby enhance joint coaptation, increase sensory stimulation of the skin, strengthen proprioceptive feedback, and ultimately contribute to pain reduction and stability. This concept is supported by biomechanical and experimental studies showing that garment–skin interface pressure and the internal stress distribution vary with the wearer’s morphology and the garment fit [16, 27, 28]. Nevertheless, there is no direct clinical evidence to show that a higher BMI alone amplifies the effectiveness of CGs; hence, this aspect remains speculative and warrants further investigation in studies of the possible interplay between body composition (i.e. lean vs. adipose mass), morphology, and pressure distribution.
The results of our pilot study suggested that patients reporting an “on-off” effect (defined as immediate symptom relief while wearing the CG and then disappearance of that relief upon removal) were more likely to respond to CG-based treatment. Although the association did not reach the usual threshold for statistical significance, there was a clear trend in which all patients experiencing an on-off effect showed an overall response to treatment. A similar, immediate improvement in balance upon donning CGs has also been reported by Benistan et al.23 in a study of patients with hEDS, which suggests that our observation is relevant. Taken as a whole, these findings suggest that the presence of an on-off effect is a potential clinical indicator of responsiveness to CG therapy. However, this hypothesis requires confirmation.
Limitations
The present study had several limitations. Firstly, given that the diagnostic terminology changed during the study period (2013–2025), patients diagnosed before 2017 were retrospectively reclassified as having hEDS or HSD, according to the updated criteria [2]. Although this approach ensured consistency, it may have led to minor misclassification bias. Secondly, the sample size was small (n = 20) and limited the study’s statistical power – especially for group comparisons. Thirdly, QoL and fatigue were not measured with standardized, validated instruments (e.g., the SF-36 questionnaire and the Fatigue Severity Scale), which reduced our ability to compare the present results with the literature data. Fourthly, the retrospective design introduced selection bias and restricted data standardization—particularly for the patients with HSD. Fifthly, the use of a composite endpoint that corresponded to at least one positive item among the four considered (a reduction in pain, a reduction in analgesic consumption, an improvement in QoL, and a reduction in fatigue) might have provided an overestimate of effectiveness.
Finally, another limitation concerns potential confounding factors. Most patients had already undergone long-term physiotherapy or rehabilitation programs before the prescription of compression garments, and these programs were usually continued during the follow-up period. Furthermore, the drug regimen might have changed for some patients. Although the rehabilitation context was therefore relatively stable at the time of CG initiation, these concomitant treatments might still have contributed to the observed reduction in symptoms, making it difficult to attribute the observed effects solely to the use of CGs. Accordingly, our findings should be interpreted as associations, rather than evidence of causality.
Perspectives
Future research should seek to clearly define the target population, i.e. those patients most likely to benefit from CGs. Profiles that might be more responsive include patients with a shorter duration of chronic pain, those with peripheral proprioceptive disturbances, and those with a higher BMI. Accurate identification of these predictive factors might guide the more judicious prescription of CGs. This aspect is especially important, given the substantial cost of CGs; in France, these garments are reimbursed by the social security system only under specific conditions and must be replaced every 6 to 24 months, due to the progressive loss of elasticity and mechanical effectiveness. Hence, in a context of healthcare resource optimization, it is essential to target patients for whom CGs are most likely to be effective.
Prospective, controlled studies are also needed, with a particular focus on HSD; the latter condition might be more prevalent than hEDS but has not been studied as extensively. These studies should incorporate validated outcome measures (e.g. pain scales, QoL questionnaires, and fatigue questionnaires), objective assessments of postural control and sensory afferents, and subgroup analyses by clinical phenotype (axial, peripheral, and visceral). Lastly, future research work should investigate the optimal daily duration of CG use, the most treatment-responsive anatomic sites, and potential synergistic effects with other proprioceptive aids and treatments (e.g., soft braces, foot orthoses, and tailored physiotherapy).