The clinical symptoms of GERD patients with LPR are more severe than those of patients with GERD alone [25]. As a first-line treatment for GERD, proton pump inhibitors (PPIs) are effective in only 60% of patients [25, 26], especially when GERD is combined with LPR. Owing to the perplexing clinical symptoms and the fear of cancer, patients with GERD combined with LPR visit gastroenterology and otolaryngology clinics for a long period for repeated examinations and drug prescriptions, which not only wastes many medical resources but also increases the economic burden on patients [27]. Therefore, it is imperative to identify the risk factors for GERD combined with LPR and to prevent it.
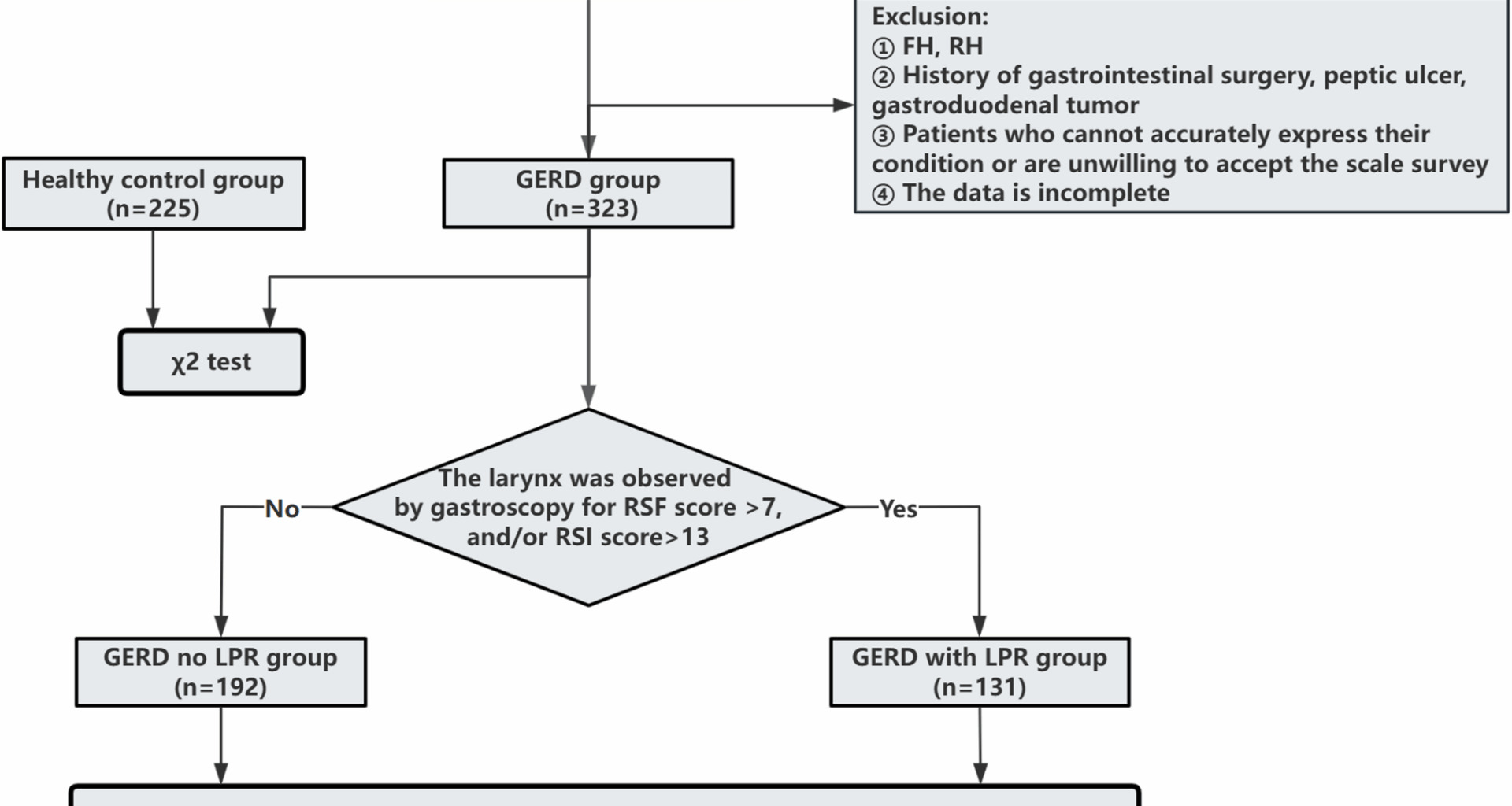
To further investigate the risk factors for GERD combined with LPR, the RSI and RFS were used for all enrolled patients, and 131 of the 323 patients with GERD had GERD combined with LPR; the prevalence rate of GERD combined with LPR was 40.56%, which was consistent with previous research results [28,29,30]. However, our operational GERD definition does not fully satisfy the Lyon consensus, and the diagnostic value of LPR remains debated. These factors may introduce misclassification. Recent advances in LPR diagnosis, such as multichannel intraluminal impedance-pH monitoring (MII-pH) [31, 32], while our model is based on readily available clinical data, future iterations should aim to incorporate these objective biomarkers for enhanced accuracy. The lifestyle habits, mental state, and other factors of patients with GERD without LPR and those with GERD combined with LPR were compared, revealing that there were differences between the two groups in terms of high-fat diet, eating too fast, overeating, supine position after meals (within 30 min), low pillow height, cervical spondylosis status, anxiety status, and other factors. Logistic regression revealed six independent correlates-low pillow height, postprandial supine, anxiety, eating too fast, overeating, and high-fat diet-collectively informing risk stratification; low pillow height showed the largest effect size in this cohort. Compared with studies conducted in Western populations, the risk factors identified here show both overlaps and distinctions. Although dietary factors are commonly reported across different regions [18, 33, 34], the strong association observed with cervical posture-related factors—particularly low pillow height—appears more prominent in our cohort. This discrepancy highlights the need for further cross-cultural research.
An unhealthy lifestyle is an important risk factor for the development of LPR. Studies have reported that smoking, alcohol consumption, high-fat diet, and lying down immediately after meals are associated with an increased risk of developing LPR [35,36,37,38]. The relationship between low pillow height and GERD combined with LPR has not been reported. According to the clinical phenomenon that patients with GERD combined with LPR often have cervical spondylosis and a low pillow height, these patients were included in the investigation of risk factors in this study. We found that a low pillow height was an important independent risk factor for the development of LPR (OR: 5.28, 95% CI: 2.384 ~ 11.508). Patients with cervical spondylosis should be required by rehabilitation doctors to adopt a low pillow height at rest. We speculate that with the use of a low-pillow height, the stomach contents of GERD patients are more likely to reach the throat through the esophagus, resulting in the occurrence of pharyngeal reflux. This association is similar to reports indicating a higher incidence of throat and laryngeal disorders in patients with spinal deformities (such as lumbar kyphosis), suggesting that there may be an association between posture and the interface between the throat and larynx [39].
Although clinical guidelines generally support that elevating the head of the bed (15 ~ 20 cm) can help reduce GERD [40, 41], this study observed in specific populations (such as patients with cervical spondylosis) that: a low pillow height (< 10 cm) was not contradictory to the significantly increased risk of GERD + LPR, and the two had different action targets. The low pillow height may increase upper airway exposure and symptoms through three pathways: (a) biomechanical changes in the cervical spine causing geometric mismatch at the pharyngoesophageal junction, reducing the baseline pressure of the upper esophageal sphincter (UES) [42]; (b) affecting the anatomical relationship between the diaphragm and the esophagus, weakening the “clamping” barrier of the lower esophageal sphincter (LES) and the diaphragm [43]; (c) neck discomfort causing sleep fragmentation, triggering more transient LES relaxations (TLESRs), and possibly affecting esophageal motility through vagus nerve regulation [44, 45].The existing evidence for elevating the head of the bed mainly focuses on “preventing gastric contents from reaching the esophagus”, while the appropriate pillow height has a greater impact on “the clearance of the upper airway and the upper barrier after reflux occurs”. Therefore, they are not mutually exclusive. We have tried to control for known confounding factors (age, BMI, cervical spondylosis, etc.), and the strong association between low pillow position and GERD + LPR remained stable, suggesting that it may be a real risk factor in a specific pathological physiological context; however, we still need to be vigilant about unmeasured confounders (severity of cervical spondylosis, sleep breathing disorders, sleep quality) and reverse causality.
In a prospective, multicenter clinical study, the rate of GERD combined with LPR in those who lay down immediately after meals was significantly higher than that in those who did not lie down immediately after meals [38]. In this prediction model, assuming a supine position within 30 min after meals (OR: 2.617, 95% CI 1.553 ~ 4.410) is another important factor influencing GERD combined with LPR, which is consistent with the results of previous studies on GERD and LPR [38]. Notably, supine position after meals was a common risk factor for GERD without LPR and GERD combined with LPR, but supine position after meals was associated with a by 2.617-fold greater risk of developing GERD combined with LPR than GERD without LPR. Shorter meals and earlier bedtimes may increase the presence of food in the stomach while lying down, which may increase gastric volume burden while supine, reduce esophageal clearance, and promote transient LES relaxations, thereby increasing reflux exposure to the hypopharynx.
In studies of risk factors for GERD and LPR, overeating, eating too fast [46, 47], and eating a high-fat diet [48,49,50] all increase reflux symptoms or frequency. Overeating and fast eating increases the pressure in the stomach caused by the retention of food in the stomach and exacerbates reflux attack. A high-fat diet significantly reduces LES stress more than a high-protein diet does, which promotes the occurrence of reflux [46]. In this study, eating too fast, overeating, and eating a high-fat diet were all factors influencing GERD without LPR and GERD combined with LPR. However, compared with that of GERD without LPR, the risk of developing GERD combined with LPR was increased 2.226 times by eating too fast, 2.185 times by overeating, and 2.05 times by eating a high-fat diet.
Studies have shown that psychological disorders such as anxiety and depression are closely related to the risk of developing LPR [30, 51, 52]. In this study, there was a significant association between anxiety status and the risk of developing LPR (OR: 2.244, 95% CI 1.144 ~ 4.404). Patients with GERD combined with LPR reportedly have higher levels of anxiety and depression than those with GERD without LPR [29], which is consistent with our findings. In one meta-analysis, up to one-third of people with GERD experienced anxiety and depression [51], and other studies reported that the higher the anxiety level was, the greater the incidence of gastroesophageal reflux was [53]. In this study, concomitant GERD and LPR status was positively associated with the risk of developing anxiety, and there may be a two-way causal relationship between anxiety and GERD combined with LPR.
Our study developed a nomogram to predict the risk of LPR in GERD patients. Several other prediction models for GERD or LPR have been developed. The COuGH RefluX score targets GERD probability in chronic laryngeal symptoms [54]; our model complements it by emphasizing lifestyle/behavioral exposures within confirmed GERD. With an AUC of 0.775, our model offers moderate discrimination; DCA suggests net benefit across risk thresholds 40%~70%. For clinical practice, our nomogram serves as a simple, non-invasive tool to stratify patients at high risk for LPR. Clinicians can use the total score to guide decisions on further diagnostic workups, such as endoscopy or pH monitoring. Patients with high pre-test probability, particularly those with modifiable risk factors identified by the nomogram (e.g., low pillow height, high fat diet), would be ideal candidates for early and targeted lifestyle interventions. Clinicians may use the nomogram to prioritize counseling on postprandial supine avoidance, diet fat content, anxiety management, and avoidance of excessively low pillow height.
We recommend maintaining neutral cervical alignment rather than a specific height, with individualized advice based on anatomy and comfort; this complements guideline‑endorsed HOBE and left‑lateral sleeping [40, 55, 56]. This intervention should be integrated into existing GERD management protocols as a first-line, low-cost strategy.
Future research should build upon our findings. First, prospective, multicenter validation studies are needed to confirm the generalizability of our nomogram. Second, mechanistic studies using high-resolution manometry and simultaneous polysomnography could elucidate the precise physiological pathways linking pillow height to reflux events. A specific hypothesis to test is whether increasing pillow height improves UES basal pressure and reduces nocturnal reflux episodes. Finally, randomized controlled trials (RCTs) are warranted to evaluate the therapeutic efficacy of pillow height modification as a formal intervention for LPR, assessing both symptomatic and objective outcomes.
Limitations
Limitations Several important limitations warrant consideration. First, the single-center, retrospective design limits generalizability and introduces potential selection and information (recall/precision) biases, as well as temporal ambiguity. Our findings from a Chinese tertiary care hospital may not apply to other populations due to genetic, cultural, and environmental differences. Asian populations may have distinct sleep ergonomics, cervical spine anatomy, and pillow preferences that influence the observed associations. Cultural dietary patterns and healthcare-seeking behaviors may also affect patient selection and symptom reporting. Given the retrospective nature, all associations should be interpreted cautiously.
Second, exposure measurement imprecision and potential misclassification should be acknowledged. Pillow height was self-reported rather than objectively measured, introducing recall/precision bias and potential nondifferential misclassification that could attenuate or distort associations. We did not standardize pillow materials or neck/cranio‑cervical angle, and musculoskeletal tolerance to different heights was not assessed.
Third, potential unmeasured confounding cannot be entirely excluded. While we controlled for major confounders including cervical spondylosis severity, sleep position preferences, medication use, and socioeconomic factors, residual confounding by sleep disorders (particularly undiagnosed sleep apnea), psychological factors, or unmeasured lifestyle variables may persist. The cross-sectional design also limits causal inference, as reverse causality (LPR symptoms influencing pillow choice) remains possible.
Fourth, the contradiction with established clinical recommendations requires cautious interpretation. Our findings may represent a population-specific phenomenon or reflect complex interactions between multiple factors not fully captured in our analysis. The mechanism we propose requires validation through prospective physiological studies.
Fifth, external validation is lacking. While internal validation through bootstrap resampling showed good model stability, performance in different populations remains unknown. The nomogram’s clinical utility requires prospective validation and comparison with existing risk assessment tools.
Finally, the relatively modest sample size may limit detection of important subgroup differences or rare risk factors. Future studies should employ larger, multicenter designs with standardized protocols to confirm these findings and explore population-specific variations.