In this cross-sectional pilot study of patients hospitalised with moderate to severe COVID-19, over half developed newly diagnosed DM after excluding those with pre-existing DM. Our findings support the hypothesis that IR, rather than insulin deficiency, is the predominant mechanism of newly diagnosed DM in this urban South African population. The TyG index, a pragmatic surrogate of IR, together with BMI, improved the diagnostic accuracy of predicting newly diagnosed DM, offering a cost-effective alternative to more complex measures such as HOMA-IR. These markers are especially practical in resource-limited settings, given their established links to T2DM and cardiovascular risk [41, 42]. The addition of BMI and selective inflammatory markers further enhanced diagnostic performance, yielding a robust model for stratifying risk of newly diagnosed DM in hospitalised COVID-19 patients.
SARS-CoV-2 has been implicated in the triggering of autoimmune responses, including T1DM, as viral infections can induce autoimmunity through mechanisms like molecular mimicry and bystander activation [8]. However, current evidence shows no significant association between SARS-CoV-2 and the presence of islet autoantibodies [8, 43]. In our sample population, pancreatic autoantibodies were predominantly negative, with only two individuals with newly diagnosed DM testing positive for anti-GAD antibodies. This suggests that autoimmune mechanisms are unlikely to be the main contributors of newly diagnosed DM in this population. These findings are consistent with national data, where type 1 DM affects approximately 32 000 of over 2.3 million people living with DM in South Africa [44, 45]. The identification of two autoimmune DM cases in our study population remains consistent with existing incidence rates and supports the need for continued surveillance and immunological profiling in COVID-19-associated dysglycaemia. Individuals with positive anti-GAD antibodies demonstrated features suggestive of IR, as reflected by elevated HOMA-IR scores, further supporting IR as the dominant mechanism in newly diagnosed DM among COVID-19 patients. In contrast, HOMA-CP proved to be an ineffective predictor of DM in this setting.
Analysis of glycaemic characteristics in our study population revealed significantly higher FPG and HbA1c levels in patients hospitalised with COVID-19 compared to healthy controls. These findings are consistent with our previous study from the first wave of the pandemic, which also identified hyperglycaemia as a key predictor of poor outcomes in COVID-19 [5]. Markers of insulin secretion and resistance, including insulin, C-peptide, HOMA-IR, HOMA-CP, and the TyG index, were significantly elevated in COVID-19 patients relative to healthy controls, with HOMA-IR and TyG index particularly increased in those with newly diagnosed DM. Collectively, these findings support IR as central to the pathogenesis of COVID-19-related hyperglycaemia. IR develops when insulin fails to act effectively on skeletal muscle, liver, and adipose tissue, typically due to post-receptor signalling defects rather than receptor abnormalities [15]. Although glucocorticoid therapy can exacerbate hyperglycaemia through both IR and β-cell dysfunction, emerging evidence suggests that IR occurs independently of steroid use [10, 16]. Severe COVID-19, particularly in patients with acute respiratory distress syndrome (ARDS), is associated with more pronounced IR and hyperglycaemia, accompanied by elevated C-peptide and amylin levels, indicative of β-cell hypersecretion rather than failure [16]. These observations highlight the metabolic disturbances associated with moderate-to-severe disease and provide mechanistic insight into one of the principal pathways contributing to newly diagnosed DM in this context.
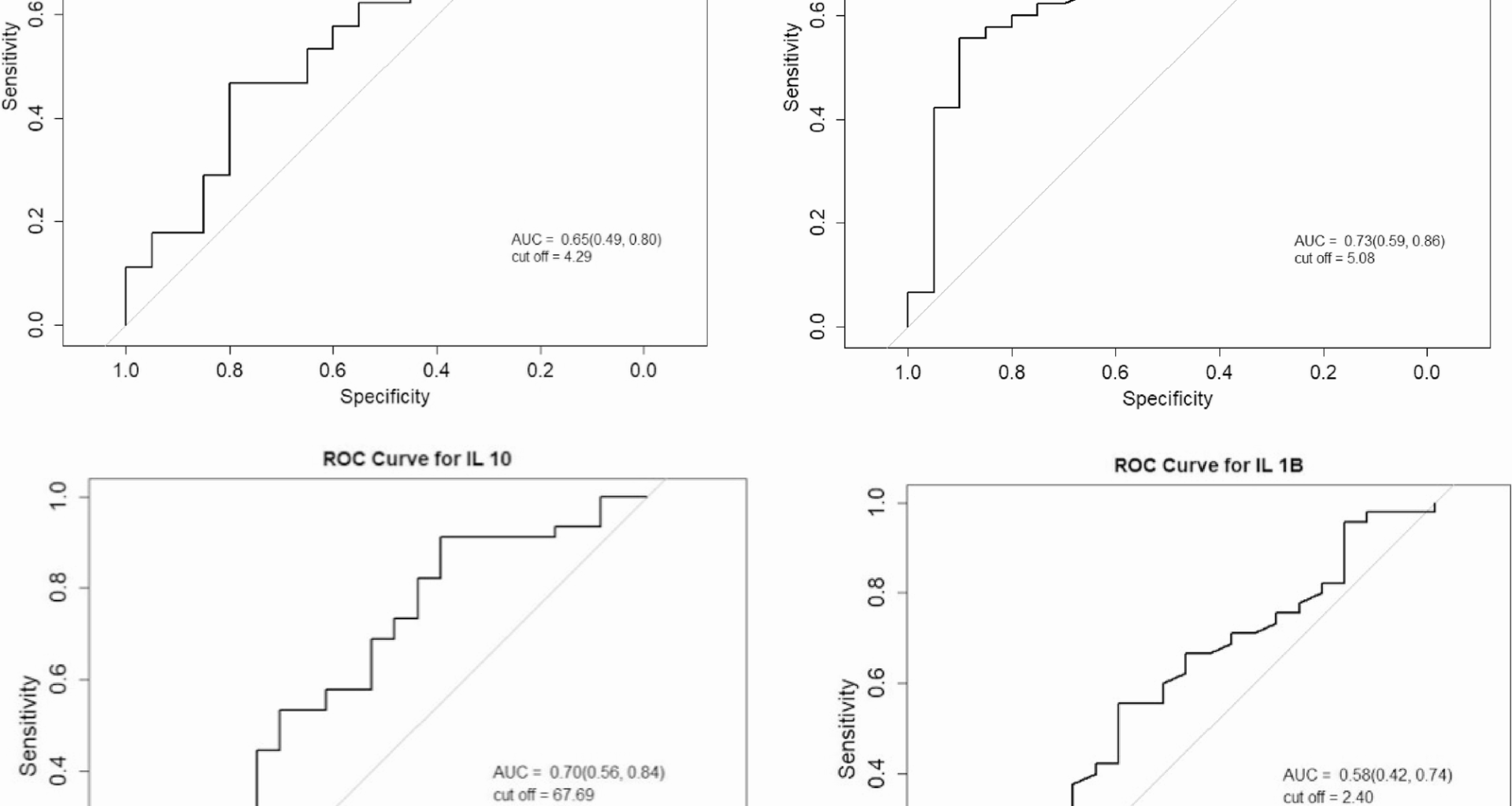
Within our study sample, a TyG index ≥5.08 was associated with 11.25 times increased odds for newly diagnosed DM. Although HOMA-IR was significantly higher in those with newly diagnosed DM compared to those without, it did not remain significant when combined with BMI in the diagnostic models. These findings highlight the potential utility of the TyG index as a simple and accessible marker for identifying individuals at risk of COVID-19 related dysglycaemia. Observational and case-control studies have similarly reported higher HOMA-IR, FPG, and insulin levels in COVID-19 patients compared to controls, supporting the association between SARS-CoV-2 infection and IR [10, 46]. However, while HOMA-IR has been associated with COVID-19 severity and long-term metabolic dysfunction, it primarily reflects hepatic rather than peripheral IR, and its predictive value for newly diagnosed DM appears limited once confounders such as BMI are considered [10, 47,48,49]. This divergence may be attributed to methodological differences and the physiological variability of fasting insulin, which can be affected by stress responses, β-cell hypersecretion, and glucocorticoid use, common features in acute illness. In contrast, the TyG index, derived from fasting triglycerides and glucose, may provide a more stable reflection of peripheral IR that may be less vulnerable to acute fluctuations. Large scale data from the PURE study demonstrated that the TyG index is significantly associated with incident T2DM and cardiovascular disease, particularly in LMIC populations, where vulnerability to IR may be heightened [22]. TyG index also correlates with future cardiovascular mortality, myocardial infarction, and stroke, highlighting the role of IR in the pathogenesis of cardiometabolic disease [22]. The TyG index is a simple and cost-effective marker that serves as an independent predictor for the onset of metabolic syndrome and T2DM in patients with COVID-19, as well as for the risk of severe illness and mortality associated with the virus [50]. Furthermore, it has been shown to independently predict metabolic syndrome, T2DM, and severe COVID-19 outcomes with greater accuracy than traditional methods like HOMA-IR [27, 41, 50]. Together, these findings highlight the TyG index as a low-cost and reliable marker for early detection of metabolic complications, particularly in resource-constrained settings. Future research should investigate the mechanisms by which COVID-19 induces IR, possibly via adiposity, inflammation, or direct β-cell effects, to refine prevention and treatment strategies.
In our study, BMI was significantly higher in patients with COVID-19 compared to healthy controls, consistent with international cohorts reporting a 1.31-fold higher pooled risk of severe COVID-19 among overweight individuals [51, 52]. The significantly higher median BMI of 37 kg/m2 in patients with newly diagnosed DM, compared to 26 kg/m2 in those without, emphasises the strong association between obesity and dysglycaemia. Furthermore, a significantly greater proportion of patients with newly diagnosed DM were obese, reinforcing obesity as a critical risk factor. Combining BMI with the TyG index further improved diagnostic performance, supporting the value of these simple, non-invasive surrogates of IR, both of which have been linked to T2DM and adverse cardiovascular outcomes [53]. Although visceral obesity is traditionally considered a superior marker of IR, recent data suggest that BMI performs comparably, with no added predictive benefit when substituting visceral fat area (VFA) for BMI [18]. Obesity, now recognised as a chronic metabolic condition, contributes to the development of IR through several mechanisms [1]. Adipose tissue acts as an active endocrine organ and expresses high levels of ACE-2, potentially facilitating SARS-CoV-2 entry [14, 54, 55]. The accumulation of excess adipose tissue also promotes inflammation via dysregulated adipokines, characterised by reduced anti-inflammatory adiponectin and increased pro-inflammatory leptin [1, 8, 16, 55]. Other proposed mechanisms include the upregulation of the RE1-silencing transcription factor (REST), which may alter metabolic pathways and contribute to long-term complications independent of pre-existing metabolic status [10, 13, 54].
The absence of pre-COVID-19 insulin sensitivity data limits our ability to exclude pre-existing IR, especially since the baseline BMI was higher in infected patients compared to healthy controls. Within the follow-up study group, patients with persistent DM had greater baseline BMI than those who achieved remission. Interestingly, despite BMI increasing at follow-up in both groups, markers of IR (HOMA-IR and TyG index) decreased, with no significant correlation observed between BMI and IR indices. This pattern aligns with studies reporting that COVID-19 can increase IR prevalence independently of BMI or obesity, with inconsistent associations between IR and adiposity [1, 10, 54, 56]. These findings underscore the multifactorial nature of COVID-19-related dysglycaemia, where systemic inflammation and immune-mediated metabolic disruption may contribute beyond adiposity alone.
In our study, inflammatory markers, including CRP, ferritin, IL-6, TNF-α and IL-1β were increased in individuals with COVID-19 compared to healthy controls. Among those with newly diagnosed DM, IL-6 and TNF-α levels were significantly higher compared to healthy controls, while IL-10 levels were significantly lower in those with COVID-19 without DM. Our study demonstrated that lower IL-10 concentrations were independently associated of newly diagnosed DM when assessed alongside IR markers, including the TyG index and BMI. Individuals with IL-10 levels ≥ 67.69 pg/mL had 88% lower odds of developing newly diagnosed DM, while elevated IL-1β was associated with a five-fold increased risk. These findings highlight the contribution of pro-inflammatory cytokine activity, alongside reduced anti-inflammatory signalling, to the pathophysiology of COVID-19-associated dysglycaemia.
These findings are consistent with studies demonstrating that COVID-19 provokes a cytokine storm that contributes to IR and hyperglycemia, partly by impairing pancreatic β-cell function, particularly in individuals with underlying metabolic risk factors [1, 8, 57]. Chronic IL-6 exposure has been shown to impair insulin sensitivity [1, 16], while TNF-α, IL-6, and MCP-1, secreted from adipose tissue and elevated in severe COVID-19 further disrupt glucose metabolism [1, 54, 57]. Elevated CRP and IL-6, in combination with reduced IL-10, have been associated with reduced insulin sensitivity [58]. Mechanistically, pro-inflammatory cytokines like TNF-α and IL-1β contribute to IR by activating protein kinase cascades that induce serine/threonine phosphorylation of IRS-1/2, impairing insulin signalling and glucose uptake [59]. IL-1β has been implicated in β-cell dysfunction and impaired glucose tolerance, with meta-analyses supporting its association with an increased risk of newly diagnosed DM, including T1DM, T2DM, and GDM [60,61,62,63]. Additionally, IL-1β may contribute to disease progression from prediabetes to overt T2DM by promoting β-cell failure and inflammation [50, 61].
Evidence suggests that an imbalance between pro-inflammatory and anti-inflammatory cytokines plays a central role in obesity and the development of IR and T2DM, however, there is limited research on the association of anti-inflammatory markers in newly diagnosed DM and COVID-19 [1, 26, 54, 57]. IL-10, a key anti-inflammatory cytokine, regulates immune responses by inhibiting pro-inflammatory mediators in macrophages and lymphocytes [26, 64]. Reduced IL-10 has been linked to IR, obesity and T2DM, with studies reporting IL-10 hypo-responsiveness or “IL-10 resistance” and reduced receptor expression in hyperglycemic states, potentially contributing to the progression of DM [26, 64, 65]. Our findings are consistent with these observations, supporting IL-10 as a potential biomarker for early detection of COVID-19 related DM and its utility in risk stratification. The lack of significant differences in other inflammatory markers between COVID-19 patients with and without newly diagnosed DM suggests that additional mechanisms, such as genetic predisposition, or variation in ACE2 and DPP4 polymorphisms, may also influence susceptibility and warrant further investigation.
Longitudinal studies report that nearly 50% of COVID-19 patients without prior DM develop new-onset hyperglycemia during acute illness, with many sustaining dysglycaemia for months post-recovery, highlighting the need for ongoing DM surveillance [13, 54, 66]. The natural history of COVID-19 associated DM remains uncertain, as most available data are derived from hospitalised patients with severe disease. In our sample population, 64% achieved remission of DM at 3-month follow-up. This aligns with previous reports showing that 41% to 79% of hospitalised COVID-19 patients, including those initially presenting with diabetic ketoacidosis, regress to normoglycemia or pre-diabetes after discharge [8, 10, 66,67,68,69]. It is important to acknowledge, however, that stress hyperglycaemia may persist beyond three months, and a longer follow-up is required to definitively distinguish transient from persistent DM. These observations suggest that COVID-19 and its treatments, such as glucocorticoids may unmask predisposition to DM or trigger transient stress hyperglycemia through exacerbation of IR. Clinically, this highlights the importance of post-discharge monitoring to avoid unnecessary long-term therapy and reduce hypoglycaemia risk.
Strengths and limitations
This study’s strengths lie in its prospective assessment of IR as a key pathophysiological mechanism for newly diagnosed DM, coupled with follow-up evaluations to assess remission. Such research is limited, particularly in resource-constrained settings, providing important insights into disease dynamics in LMICs. However, the single-centre design, potential selection bias due to non-randomisation, modest sample size, lack of pre-COVID-19 baseline data, and the potential contribution of comorbidities to inflammation may restrict the generalisability of the findings and complicate causal inference. The predominantly Black African sample population, with a high prevalence of obesity among newly diagnosed DM cases, reflects the real-world demographic and disease burden locally but may reduce applicability to more diverse populations. Inclusion of both general ward and ICU patients introduces clinical heterogeneity. However, all participants had moderate to severe COVID-19 and were enrolled at the point of emergency department presentation, with sampling conducted before ICU-specific interventions or treatment initiation, thereby minimising treatment-related confounding. As the study was conducted before the widespread vaccine rollout, the impact of COVID-19 vaccination on disease severity and metabolic outcomes could not be fully assessed. Only 9% of individuals with newly diagnosed DM and 10% of those without DM had received at least one vaccine dose prior to admission, with no significant difference in vaccination status between subgroups, making it unlikely to have influenced or biased the results.
Several factors can influence HbA1c measurements, including variations in haemoglobin, differences in glycation rates, and changes in erythrocyte lifespan related to various medical conditions [38]. Consequently, some individuals may present with normal HbA1c levels despite having elevated glucose levels or vice versa [38]. However, as haemoglobin levels in our study remained within normal reference ranges across groups, this is unlikely to have significantly biased the interpretation of HbA1c. Another potential limitation of our study lies in how we assessed IR. While the TyG index is a well-validated and pragmatic tool, outcomes may differ if alternative methods such as the hyperinsulinemic-euglycemic clamp or Matsuda index were used. Additionally, although we referenced the commonly used HOMA-IR threshold of ≥ 2.5, this lacks universal standardisation, and its interpretation should consider population-specific validation. Similarly, the TyG index cutoff for IR may vary across ethnic groups, and further validation in African populations is warranted to establish appropriate thresholds. The reliance on a single follow-up after three months limited our ability to evaluate long-term remission, and no serial HbA1c data were collected thereafter to assess for recurrence or delayed conversion to DM. Although predictive diagnostic modelling identified factors associated with newly diagnosed DM in COVID-19, it was based on baseline cross-sectional data; therefore, temporality and causal inference cannot be established, and validation in larger prospective cohorts is required. Furthermore, the TyG index may be influenced by acute stress-related hyperglycaemia and lipid fluctuations in severe illness, and results should be interpreted with this context in mind. Validation in larger, longitudinal cohorts, including post-acute and recovery phases, will be important to confirm its utility. Finally, the wide 95% confidence intervals in our regression models likely reflect the modest sample size and number of outcome-positive cases. Although model complexity was minimised to avoid overfitting, the small number of patients remains a key limitation, and this should be considered when interpreting the generalisability of the findings.
Recommendations and future studies
This pilot study highlights the need for larger, longitudinal studies with serial monitoring of glycaemic indices and diabetes-related complications [38]. To build on these findings, future research should focus on larger cohorts with extended follow-up periods to accurately assess the long-term outcomes associated with newly diagnosed DM in COVID-19 patients. The TyG-BMI index, a simple and reliable marker reflecting both IR and obesity, has recently shown superior predictive value for 365-day mortality and major cardiovascular events in severe coronary heart disease [42], highlighting the need for future studies to validate its accuracy and utility in diverse populations like ours, for improved risk stratification and individualised care. Investigating interventions aimed at reducing IR in these individuals will be essential for enhancing prevention and management strategies. Additionally, developing community-based programs that address both obesity and metabolic health post-COVID-19 could be beneficial in reducing the incidence of DM in vulnerable populations. Ongoing studies within our study population are evaluating ACE-2 and DPP4 concentrations, as well as genetic variants such as single-nucleotide polymorphisms (SNPs), which may influence susceptibility to COVID-19-related dysglycemia and metabolic dysfunction persistence. Understanding these polymorphisms could elucidate host response variability and inform targeted prevention strategies. Future directions should also include larger randomised controlled trials and the inclusion of additional metabolic and inflammatory markers to refine and validate predictive diagnostic models.