In a breakthrough that redefines both speed and clinical potential, a new world record for the fastest human whole genome sequencing has been set.
KamranAydinov on Freepik
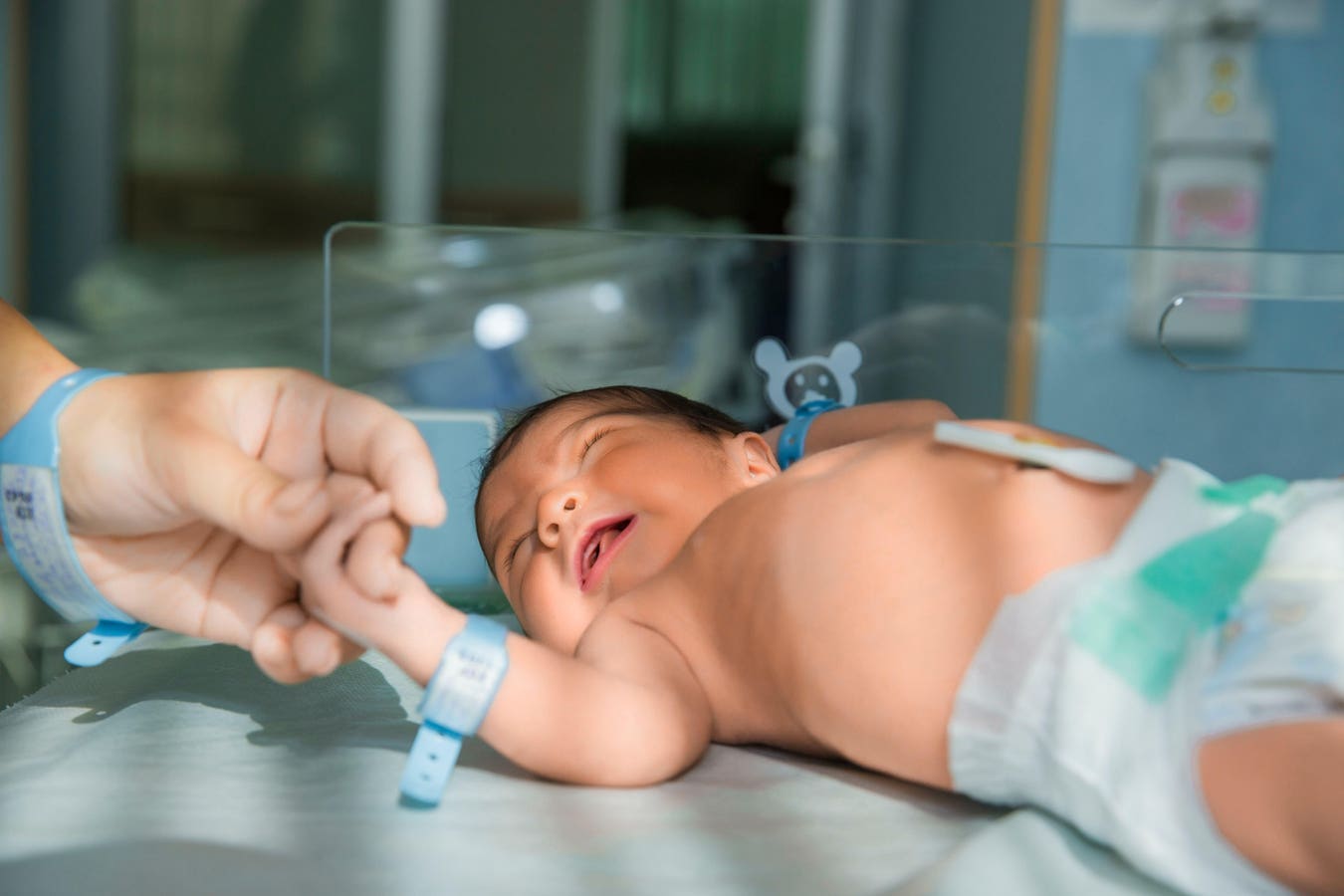
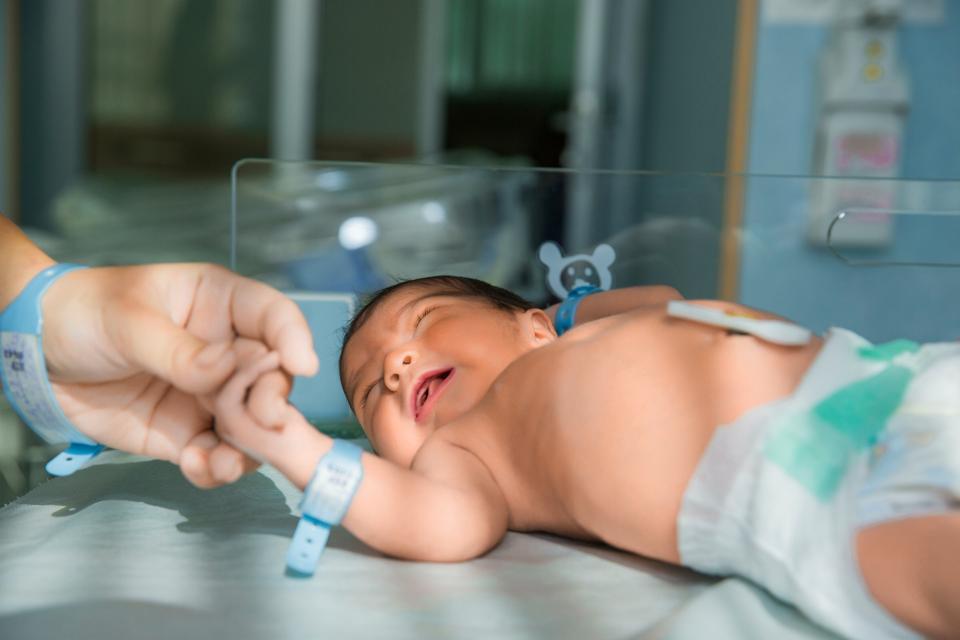
Think of all the things that can be done in four hours: a long flight, a double feature, or now, the sequencing of an entire human genome. In a breakthrough that redefines both speed and clinical potential, a new world record for the fastest human whole genome sequencing has been set. The team, led by researchers from Boston Children’s Hospital, Broad Clinical Labs and Roche Sequencing Solutions, completed the sequencing and analysis of a full human genome in less than four hours — a pace that brings the vision described in Destiny’s Child No Longer: Rewriting Genetic Fate closer to reality. That vision, in which every newborn could undergo comprehensive genomic screening to transform the uncertainties of genetic inheritance into opportunities for early intervention, is now emerging from theory into practice.
A Record Beyond the Stopwatch
Published in the New England Journal of Medicine, the study represents a leap from the research lab into real-world hospital workflows. This new method holds an official Guinness World Record for speed, but its implications go far beyond setting records. The process was designed with one urgent application in mind: critically ill newborns in neonatal intensive care units (NICUs), where hours can determine whether a child receives life-saving care or undergoes unnecessary procedures.
As Dr. Monica Wojcik, pediatrician and geneticist at Boston Children’s Hospital and the study’s lead author, explained, “Our pilot simulates a workflow through which we could feasibly send a genome sequencing sample from a baby in the morning and have the diagnosis and report ready by the afternoon.”In that context, sequencing speed is not a laboratory challenge; it’s a matter of clinical urgency.
The Technology Behind the Breakthrough
At the heart of the achievement is Roche’s new Sequencing by Expansion (SBX) technology, a biochemical process that temporarily expands DNA molecules to make them easier to read during analysis. Conventional next-generation sequencing techniques pause for data processing; SBX allows information to be analyzed continuously as it is generated. This real-time feedback loop reduces turnaround time without compromising accuracy.
The workflow integrates sample preparation, sequencing and bioinformatic analysis into a process that can be used in standard hospital laboratories. By improving each step, the team produced results that can be available within the same workday as other emergency diagnostics.
Transforming Critical Care Practice
This four-hour sequencing workflow is more than a technical milestone; it marks a shift toward integrating genomics into acute clinical decision-making. In neonatal intensive care, where roughly half of admissions involve congenital or genetic components, the availability of same-day genomic data could change how physicians manage treatment for rare disorders, metabolic diseases, or unexplained organ failures.
Traditional genetic testing usually takes days or weeks. These delays can lead to longer hospital stays or missed opportunities for treatment. The new sequencing approach allows for real-time precision medicine, making genome data available for immediate clinical use.
From Proof of Concept to Clinical Routine
While the record-setting performance is already being hailed as a landmark, it’s acknowledged that widespread implementation will require additional validation. Next steps involve scaling the process across larger patient cohorts, ensuring reproducibility across laboratories and addressing regulatory standards for clinical-grade genomic testing.
As sequencing technologies mature, cost barriers are falling and hardware is becoming more compact and automated. The collaboration demonstrates that what was once the domain of elite research centers can soon be standard practice at major hospitals, perhaps even expanding to community healthcare systems over time. The researchers are also exploring the application of ultra-rapid sequencing for adult intensive care units, oncology and infectious disease diagnostics, where rapid genomic insights could guide therapy selection or outbreak containment.
A Glimpse of the Genomic Future
Two decades after the first human genome was mapped at a cost of nearly $3 billion, the ability to decode the entire genetic blueprint in under four hours, potentially within the same shift that a child is admitted to the NICU, marks a stunning convergence of computing, chemistry and clinical care.
This advance aligns with the broader vision outlined in the book I coauthored, Destiny’s Child No Longer: Rewriting Genetic Fate. In these publications, a future is envisioned where every newborn undergoes comprehensive genomic screening, transforming the unpredictable lottery of genetic inheritance into an opportunity for early intervention and personalized care.
The four-hour sequencing development makes this vision more achievable. Programs in the United Kingdom and Florida are already starting universal newborn genomic screening. The NHS has committed £650 million to provide whole genome sequencing to every newborn in England by 2030. These initiatives show that this approach is becoming part of clinical practice.
The next time you think about what can be accomplished in four hours, add “reading the book of life” to the list and, perhaps more importantly, add “preventing a lifetime of suffering through early genetic intervention.”