FT-IR analysis
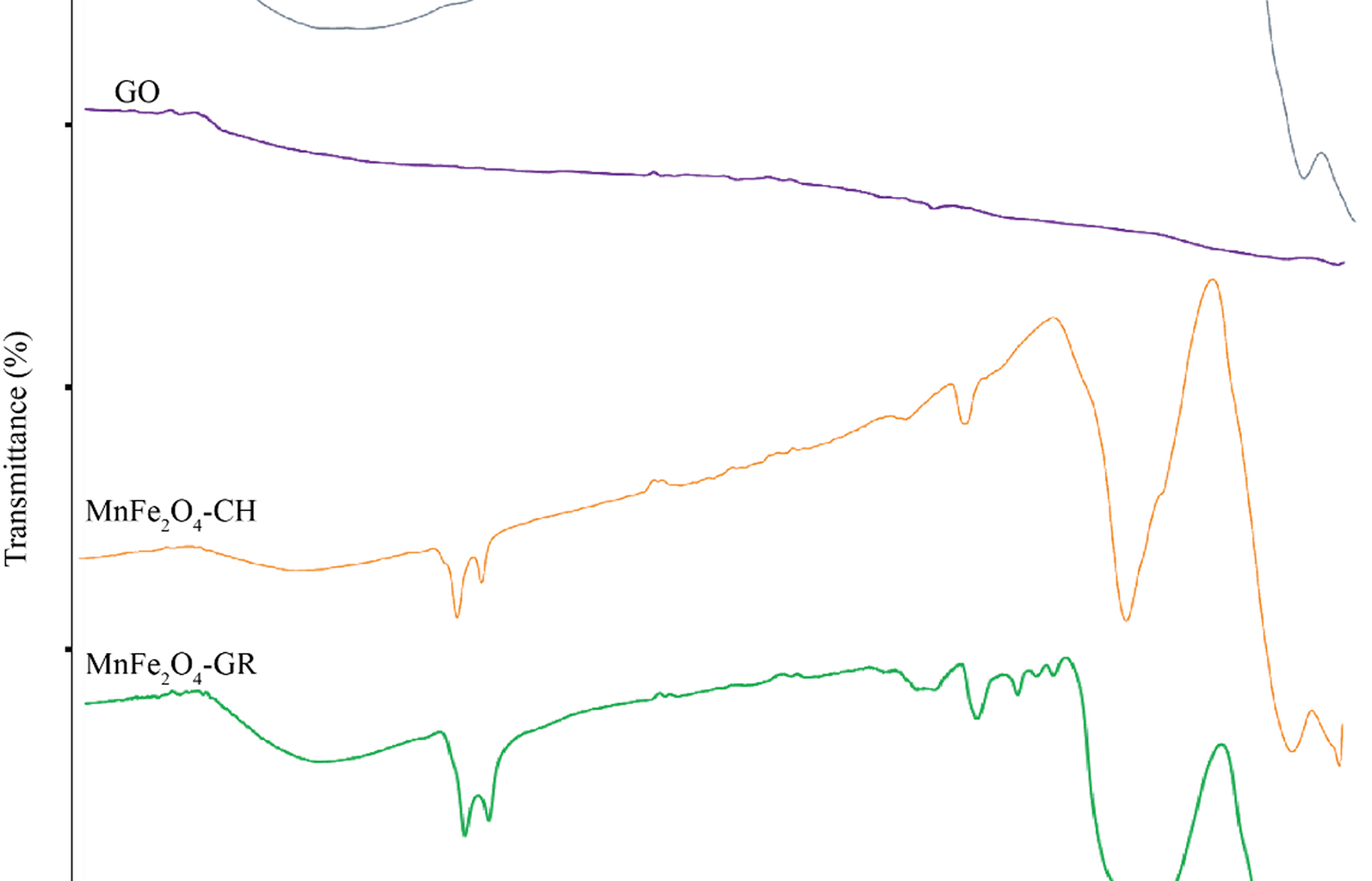
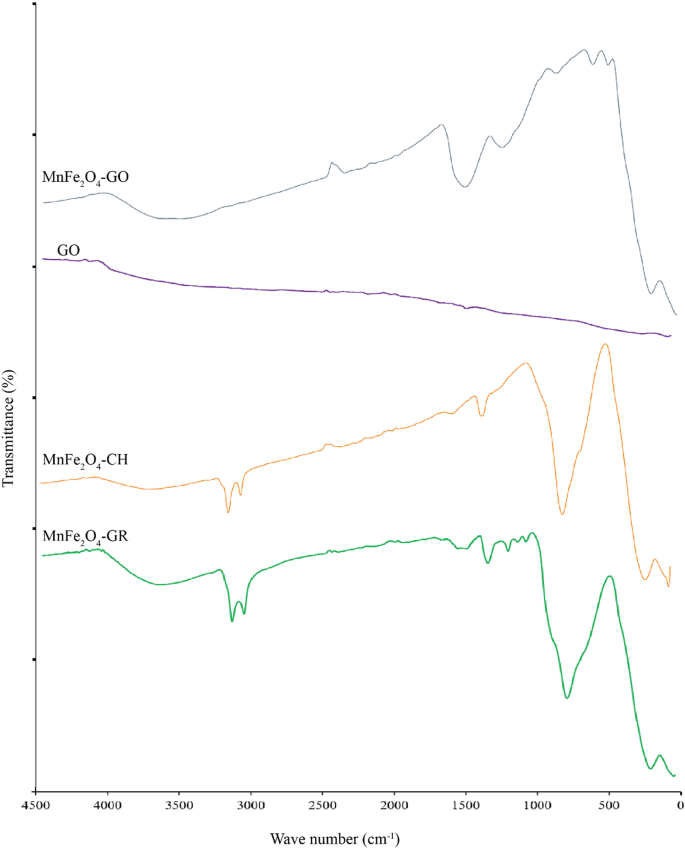
The FTIR spectra were analyzed to determine the chemical functional groups present in MnFe₂O₄ NPs synthesized by both green and chemical routes, GO, and their composite (MnFe₂O₄/GO). As shown in Fig. 1, each sample exhibits characteristic absorption peaks corresponding to its unique composition and method of synthesis.
FTIR spectra of MnFe₂O₄ nanoparticles synthesized through chemical (MnFe₂O₄-CH) and green (MnFe₂O₄-GR) methods, GO, and the MnFe₂O₄/GO nanocomposite
In the spectrum of green-synthesized MnFe₂O₄ (MnFe₂O₄-GR), a broad absorption band near 3390 cm⁻¹ corresponds to the O–H stretching vibrations, indicative of hydroxyl groups originating from absorbed water molecules or phenolic compounds in the nettle extract. The bands at ~ 1400–1450 cm⁻¹ are assigned to symmetric and asymmetric stretching of –COO⁻ (carboxylate) groups, confirming the presence of organic molecules from the plant extract, which play a role in stabilizing and functionalizing the NPs. Strong peaks observed in the region 500–700 cm⁻¹ are attributed to Fe–O stretching vibrations, confirming the formation of a spinel ferrite structure, where Fe³⁺ ions occupy both tetrahedral and octahedral sites. The chemically synthesized MnFe₂O₄ (MnFe₂O₄-CH) spectrum also displays O–H (~ 3400 cm⁻¹) and Fe–O (500–700 cm⁻¹) bands, but lacks peaks in the 1400–1500 cm⁻¹ region, indicating the absence of organic surface groups. This reflects the purity of the product and the lack of biological capping agents in conventional synthesis [25,26,27].
For GO, characteristic peaks are observed at 3400 cm⁻¹ (O–H stretching), 1720 cm⁻¹ (C = O stretching of carboxylic acid), 1620 cm⁻¹ (C = C stretching of unoxidized graphitic domains), and 1050–1250 cm⁻¹ (C–O–C and C–OH stretching). These oxygen-containing functional groups enable strong interaction with metal oxide NPs and improve dispersibility in aqueous media [28, 29]. The MnFe₂O₄/GO nanocomposite spectrum shows a combination of the characteristic peaks of both GO and MnFe₂O₄. The O–H and Fe–O peaks remain visible, with slight shifts in peak positions and intensities, likely due to chemical interactions and bonding between MnFe₂O₄ NPs and GO sheets. The persistence of C = O and C–O–C bands also supports successful anchoring of NPs onto the GO surface [30, 31]. These observations confirm not only the successful synthesis of each component but also the functional integration of MnFe₂O₄ onto the GO matrix, which is critical for biomedical applications where surface chemistry affects biological interactions. The observed FTIR bands and their chemical assignments are summarized in Table 1, highlighting functional groups relevant to both NPs stabilization and composite integration.
Table 1 FTIR peak assignments and functional group interpretationXRD analysis
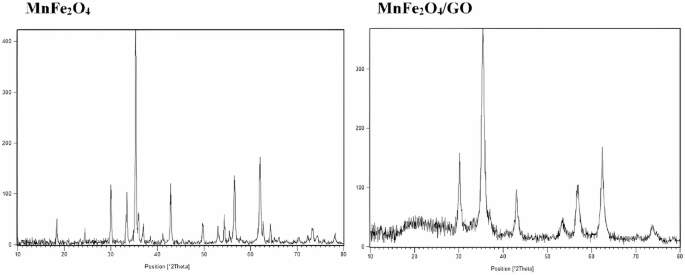
The structural properties of the synthesized MnFe₂O₄ NPs via green synthesis (MnFe₂O₄-GR) and the MnFe₂O₄/GO nanocomposite were investigated through X-ray diffraction (XRD) analysis (Fig. 2). The XRD pattern of MnFe₂O₄-GR displays sharp and well-defined peaks at 2θ values of 30.2°, 35.6°, 43.3°, 53.6°, 57.2°, and 62.8°, corresponding respectively to the (220), (311), (400), (422), (511), and (440) planes of a cubic spinel ferrite structure. These reflections match well with standard JCPDS card No. 74-2403, confirming the successful formation of single-phase MnFe₂O₄ with high crystallinity and no observable secondary or impurity phases [36,37,38]. To estimate the average crystallite size (D), the Debye–Scherrer equation was applied:
XRD patterns of MnFe₂O₄ nanoparticles (left) and MnFe₂O₄/GO nanocomposite (right)
$$\:D=\frac{K\lambda\:}{\beta\:cos\theta\:}$$
where K is the shape factor (taken as 0.9), λ is the X-ray wavelength (1.5406 Å for Cu Kα), β is the full width at half maximum (FWHM) of the most intense peak (311), and θ is the Bragg angle. The calculated crystallite size of MnFe₂O₄-GR was approximately 12 nm, consistent with nanoscale dimensions necessary for biomedical applications.
The XRD pattern of the MnFe₂O₄/GO composite shows the characteristic peaks of the spinel MnFe₂O₄ phase, confirming that the core structure of the NPs remains intact upon incorporation into GO. A broad diffraction feature around 2θ ≈ 10.5° is additionally observed, corresponding to the (001) plane of graphene oxide, indicating successful inclusion of GO sheets. A noticeable reduction in the intensity of MnFe₂O₄ peaks in the composite is evident, likely due to decreased crystallinity or diffraction shielding by the GO matrix. This may also reflect partial structural disorder caused by NPs anchoring on the GO surface [32, 33].
The average crystallite size for the MnFe₂O₄/GO sample, calculated using the same (311) peak, was approximately 16.7 nm, slightly larger than that of MnFe₂O₄-GR. This increase may be attributed to the aggregation or mild recrystallization during composite formation, although no peak broadening indicative of strain or significant lattice distortion was observed. Importantly, no new peaks or foreign phase reflections are detected in the composite, indicating excellent phase purity in both systems. Notably, no significant lattice parameter shift or peak position change was observed between MnFe₂O₄-GR and MnFe₂O₄/GO. This suggests that the incorporation of GO does not lead to doping or substitutional changes in the MnFe₂O₄ crystal lattice. The overall results confirm that the structural integrity and phase purity of the MnFe₂O₄ NPs are preserved, and the composite formation does not compromise their crystalline framework. These findings validate the successful green synthesis and structural compatibility of MnFe₂O₄ and GO in the nanocomposite, making them highly promising for further biomedical applications requiring pure, stable, and nanostructured materials.
DLS analysis
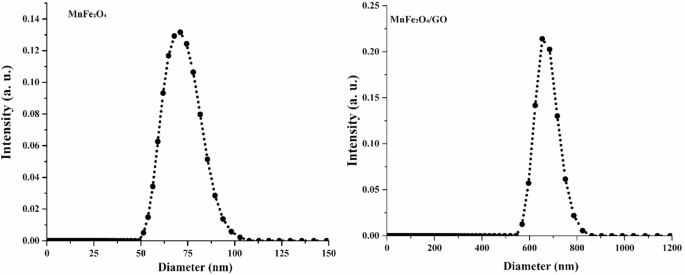
To evaluate the colloidal properties and dispersion behavior of the synthesized nanomaterials under physiologically relevant aqueous conditions, dynamic light scattering (DLS) analysis was conducted. As shown in Fig. 3, the MnFe₂O₄ NPs exhibited a narrow hydrodynamic size distribution centered around ~ 75 nm, indicating uniform dispersion and minimal aggregation in aqueous suspension. In contrast, the MnFe₂O₄/GO nanocomposite showed a broader size distribution with a dominant peak between 650 and 700 nm. This increase in hydrodynamic size can be attributed to the lateral extension of GO sheets and their interaction with the magnetic NPs, which enhances the composite’s effective particle size in solution.
Dynamic light scattering (DLS) analysis of MnFe₂O₄ nanoparticles and MnFe₂O₄/GO nanocomposites
The surface charge characteristics of the samples were also consistent with colloidal stability requirements. MnFe₂O₄ NPs exhibited a moderately negative surface charge (~ − 27.4 mV), while the MnFe₂O₄/GO composite showed a slightly more negative zeta potential (~ − 31.6 mV), which is likely due to the presence of carboxyl and hydroxyl functional groups on the GO surface. Zeta potential values in this range (|ζ| >25 mV) generally indicate sufficient electrostatic repulsion to prevent NPs aggregation, promoting colloidal stability in aqueous environments [34, 35].
The long-term stability of the dispersions was further assessed by storing the NPs suspensions in phosphate-buffered saline (PBS) at 4 °C for up to 14 days. No significant sedimentation, turbidity changes, or visible aggregation were observed during this period, indicating that both MnFe₂O₄ and MnFe₂O₄/GO formulations retain good dispersion stability under storage conditions relevant to biomedical use. Moreover, no appreciable changes were detected in either the hydrodynamic particle size or the zeta potential values throughout the storage period, confirming the structural integrity and electrostatic stability of the NPs suspensions. These findings support the potential applicability of these nanomaterials in biological systems, where consistent colloidal behavior is critical for effective delivery, biodistribution, and cellular interaction.
SEM and EDX analysis
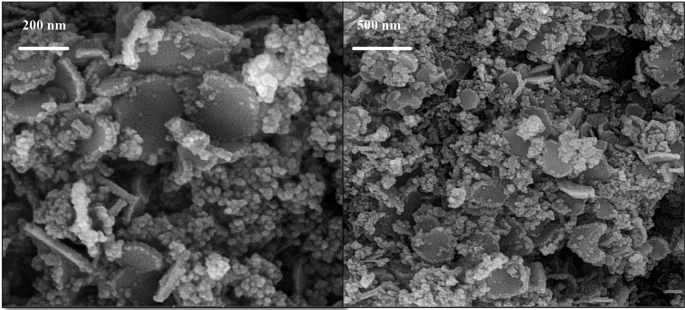
The surface structure and elemental makeup of MnFe₂O₄ NPs were examined through SEM and EDX techniques. The SEM images (Fig. 4) reveal that the MnFe₂O₄ NPs exhibit an aggregated and roughly spherical morphology with a relatively uniform distribution. The particles are nanoscale in size, with some aggregation likely resulting from the magnetic interactions between the NPs. The high-magnification image provides a detailed view of the surface texture, showing the presence of fine structures and individual particles within the aggregates. These observations confirm that the MnFe₂O₄ NPs are successfully synthesized and exhibit a morphology typical of magnetic NPs prepared through a green synthesis method.
SEM images of MnFe₂O₄/GO nanoparticles at different magnifications
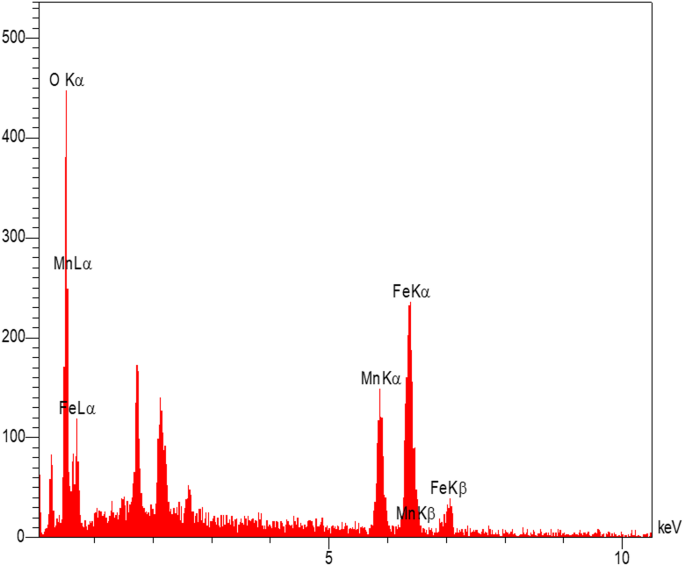
The EDX spectrum confirms the elemental composition of the MnFe₂O₄ NPs (Fig. 5). The prominent peaks corresponding to manganese (Mn), iron (Fe), and oxygen (O) indicate the formation of MnFe₂O₄, consistent with the expected stoichiometry of the spinel ferrite structure. The absence of significant peaks for other elements demonstrates the high purity of the NPs, with no detectable contamination from precursor materials or other impurities. The presence of oxygen further supports the formation of a metal oxide structure. Overall, the SEM analysis highlights the nanoscale morphology and aggregation behavior of the MnFe₂O₄ NPs, while the EDX results confirm their elemental composition and purity. These findings are in alignment with the intended synthesis of MnFe₂O₄ NPs and support their suitability for subsequent composite formation and biomedical applications.
EDX spectrum of MnFe₂O₄/GO nanoparticles showing the elemental composition
Examination of nanoparticles using transmission electron microscopy (TEM)
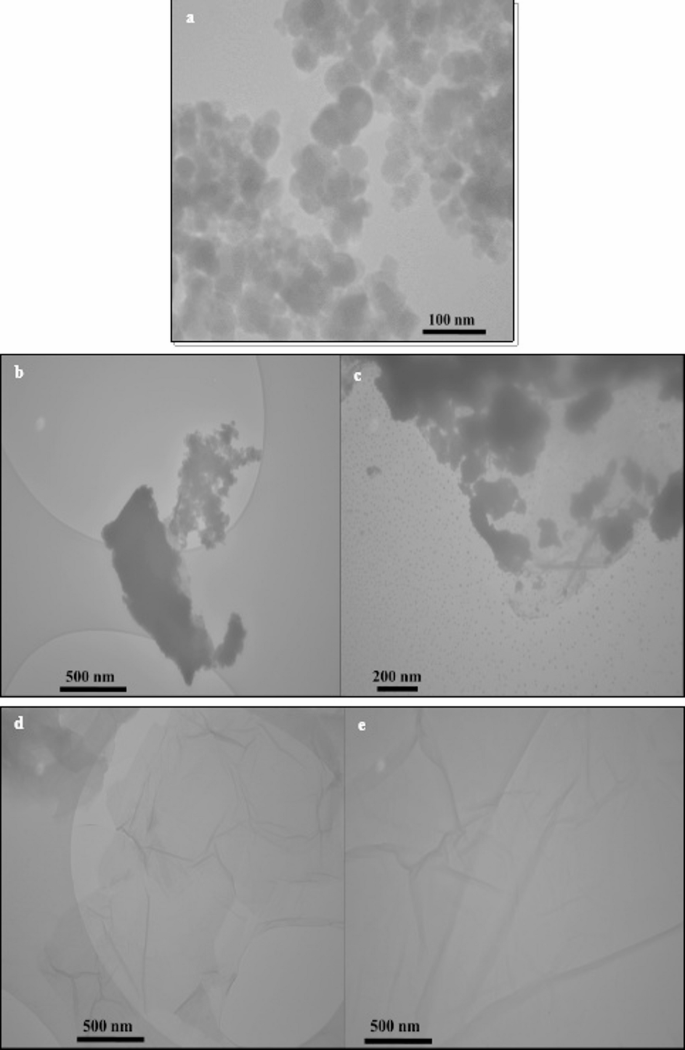
A Transmission Electron Microscopy (TEM) investigation was conducted to assess the structural characteristics, morphological features, and distribution patterns of the synthesized MnFe₂O₄ NPs, GO, and the MnFe₂O₄/GO nanocomposites. Each material displayed distinct characteristics, confirming their successful synthesis and structural integrity. The TEM images of MnFe₂O₄ NPs (Fig. 6a) synthesized through the green method reveal roughly spherical NPs with a narrow size distribution. The particles exhibit some degree of aggregation, which is common for magnetic NPs due to strong magnetic dipole-dipole interactions. The individual particle sizes are in the nanoscale range, consistent with the crystallite size calculated from the XRD analysis, which confirms the formation of MnFe₂O₄ NPs with a uniform morphology and structural integrity. For GO NPs, the TEM images (Fig. 6b-c) display thin, transparent, and wrinkled sheet-like structures, characteristic of exfoliated GO layers. The sheets appear to overlap in some regions, which is consistent with the layered nature of GO. The transparency of the sheets indicates their nanoscale thickness, and the observed wrinkles are a result of mechanical flexibility and distortions caused during synthesis and drying processes. These characteristics validate the effective production of graphene oxide, demonstrating a significant level of layer separation. For the MnFe₂O₄/GO nanocomposites (Fig. 6d-e), the TEM images provide clear evidence of the successful deposition of MnFe₂O₄ NPs onto the GO nanosheets. The NPs are uniformly distributed over the GO surface, indicating a strong interaction between MnFe₂O₄ and GO throughout the synthesis process, which facilitated their effective integration. The wrinkled structure of GO remains visible, indicating that the layered nature of GO is retained in the composite. The dispersion of NPs across graphene oxide sheets significantly increases the overall surface area and improves the functional characteristics of the composite, rendering it highly effective for applications like targeted drug delivery and cancer therapy. Therefore, the TEM analysis confirms the successful synthesis of MnFe₂O₄ NPs, graphene oxide, and their composite. The structural features observed in the images align with the expected characteristics of each material, supporting their suitability for further investigations and biomedical applications.
TEM images of (a) MnFe₂O₄ nanoparticles, (b, c) GO, and (d, e) MnFe₂O₄/GO nanocomposite with corresponding nanometer-scale bars
The magnetic characteristics of mnfe₂o₄ nanoparticles
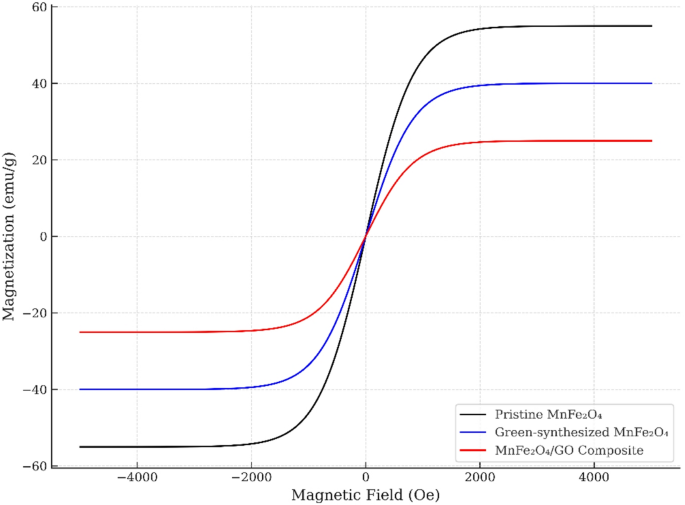
The magnetic behavior of the pristine MnFe₂O₄, green-synthesized MnFe₂O₄ (MnFe₂O₄-GR), and MnFe₂O₄/GO nanocomposite was evaluated using a vibrating sample magnetometer (VSM) at room temperature, and the resulting M–H curves are shown in Fig. 7. All samples exhibit narrow hysteresis loops with negligible coercivity and remanence, indicative of superparamagnetic behavior, which is desirable for biomedical applications due to the absence of remanent magnetization after removing the external magnetic field. The saturation magnetization (Ms) values were found to be approximately 55 emu/g for pristine MnFe₂O₄, 40 emu/g for MnFe₂O₄-GR, and 25 emu/g for the MnFe₂O₄/GO nanocomposite. The reduction in Ms in the green-synthesized sample compared to pristine MnFe₂O₄ can be attributed to surface disorder, smaller particle size, and possible cation redistribution induced by the green synthesis process [36,37,38,39]. Further reduction in Ms upon GO incorporation is likely due to the non-magnetic nature of graphene oxide and partial surface shielding of magnetic domains by the GO sheets [40, 41]. Nevertheless, all values remain within the acceptable range for magnetic biomedical applications.
M–H curves of pristine MnFe₂O₄, green-synthesized MnFe₂O₄, and MnFe₂O₄/GO nanocomposite
The coercivity (Hc) values of all samples were below 50 Oe, and the loops show a sharp rise in magnetization at low fields, which further confirms the high magnetic susceptibility and rapid response of the NPs to external magnetic fields [40, 42]. These properties are highly suitable for magnetic drug targeting, where responsiveness to low magnetic fields ensures effective navigation in vivo, and for magnetic hyperthermia, where the heating efficiency correlates with the superparamagnetic relaxation behavior.
Evaluation of nanoparticle-induced cytotoxicity in HeLa cell lines using the MTT assay
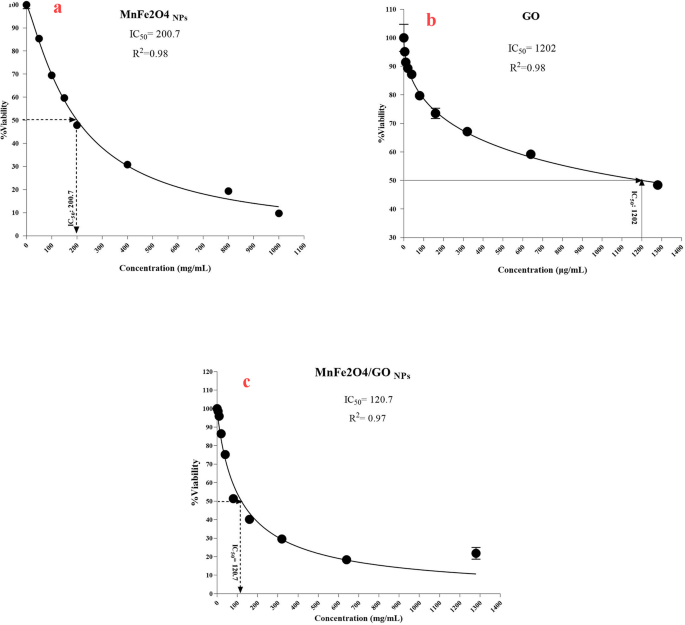
The cytotoxic effects of MnFe₂O₄ NPs, GO, and their MnFe₂O₄/GO nanocomposite were assessed on HeLa cells using the MTT assay to evaluate their potential anticancer activity (Fig. 8). Cell viability was measured across a range of NPs concentrations, and the half-maximal inhibitory concentration (IC₅₀) was determined for each formulation. The MnFe₂O₄ NPs (Fig. 8a) displayed notable cytotoxicity with an IC₅₀ of 200.7 µg/mL, suggesting a strong concentration-dependent inhibition of HeLa cell proliferation. This cytotoxic response is likely attributed to the generation of reactive oxygen species (ROS) and the induction of mitochondrial dysfunction, as observed in similar magnetic NPs, which may lead to apoptotic pathways being triggered in cancer cells. In contrast, GO alone (Fig. 8b) exhibited significantly lower toxicity, with an IC₅₀ of 1202 µg/mL, indicating its relative biocompatibility at low concentrations. However, at higher concentrations, GO has been reported to disrupt cellular membranes and induce oxidative stress, both of which may contribute to its mild cytotoxic effect.
Dose-response curves showing the cytotoxic effects of (a) MnFe₂O₄ nanoparticles, (b) GO, and (c) MnFe₂O₄/GO nanocomposite on HeLa cell viability, as determined by the MTT assay
The MnFe₂O₄/GO nanocomposite (Fig. 8c) demonstrated superior cytotoxicity, with a markedly lower IC₅₀ of 120.7 µg/mL. This enhanced cytotoxic effect can be attributed to a synergistic mechanism involving multiple factors: the magnetic core (MnFe₂O₄) likely facilitates improved endocytic uptake via magnetic interactions and cellular internalization pathways, while the GO matrix provides a large surface area that enhances cellular contact and delivery [43, 44]. Furthermore, both components are known to contribute to elevated intracellular ROS levels, which can trigger oxidative damage, mitochondrial membrane potential loss, and apoptosis [45, 46]. Although direct ROS quantification or endocytosis assays were not performed in this study, the significantly lower IC₅₀ of the composite compared to individual components supports a mechanism involving both enhanced intracellular delivery and ROS-mediated cytotoxicity. These findings emphasize the potential of MnFe₂O₄/GO nanocomposites as effective therapeutic agents in cervical cancer treatment by leveraging dual-action mechanisms [47,48,49].
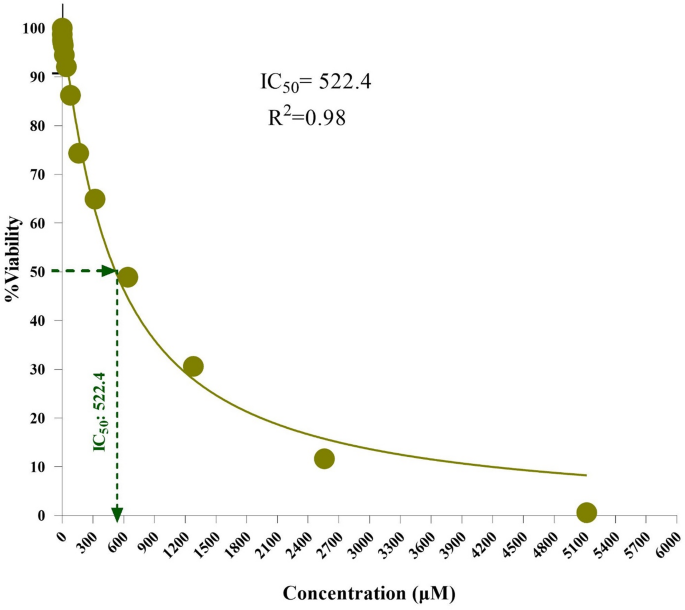
We addressed the sensitivity of normal cells in the context of our study. We observed no cytotoxic effects on HFF-2 cells in the treatment groups (Fig. 9). We emphasized that this does not necessarily imply a complete lack of sensitivity in all normal cells. We clarified that differential toxicity can occur and discuss the implications of our findings regarding normal cell responses (Fig. 9).
Cell viability assay of MnFe₂O₄/GO nanocomposites against HFF-2 cell line
Effects of nanoparticles on apoptosis-related gene expression
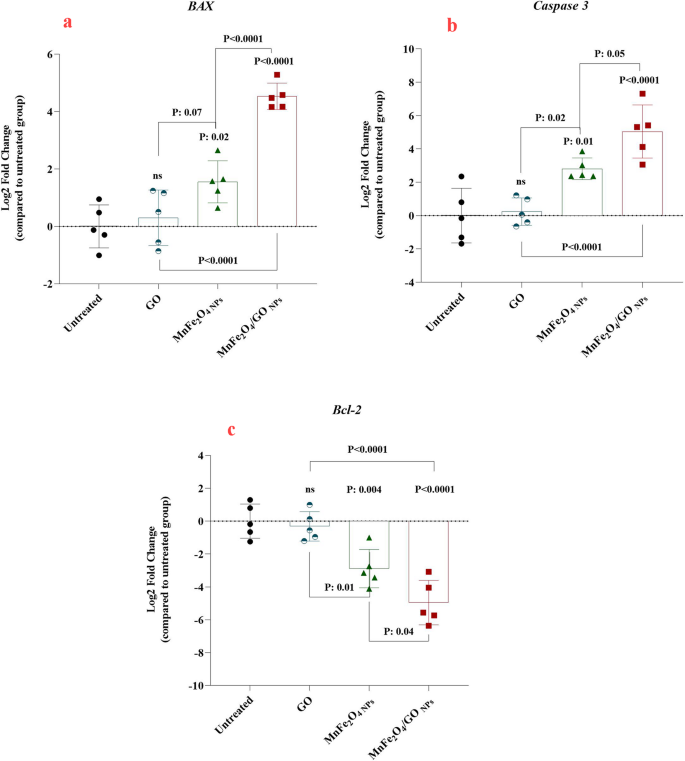
The effects of MnFe₂O₄ NPs, GO, and their composite (MnFe₂O₄/GO NPs) on apoptosis-related gene expression in HeLa cells was conducted by examining the expression patterns of BAX, Caspase-3, and Bcl-2 (Fig. 10). These genes play a crucial role in controlling the process of programmed cell death. BAX and Caspase-3 facilitate apoptosis by activating cell death pathways, whereas Bcl-2 functions as a protective factor, preventing apoptosis from occurring. The findings reveal a notable increase in the expression of genes associated with programmed cell death, specifically BAX and Caspase-3, while simultaneously demonstrating a marked suppression of the Bcl-2 gene, which is known for its anti-apoptotic function. This regulatory shift occurs as a direct consequence of exposure to MnFe₂O₄/GO NPs. For BAX (Fig. 10a), the expression level was markedly increased in the MnFe₂O₄/GO-treated group compared to untreated cells and cells treated with MnFe₂O₄ NPs or GO alone. The MnFe₂O₄/GO-treated group exhibited a fold change that was statistically significant (P < 0.0001), highlighting the synergistic effect of the composite in activating apoptotic pathways. Similarly, Caspase-3 expression was significantly higher in the MnFe₂O₄/GO-treated group, with a similar trend observed when compared to the individual components (Fig. 10b). The results suggest that the composite induces enhanced activation of caspase-mediated apoptosis, which is critical for cell death in cancer therapy. Conversely, the levels of the Bcl-2 gene, which plays a role in preventing apoptosis (Fig. 10c), were markedly diminished in cells exposed to MnFe₂O₄/GO NPs. This suppression of Bcl-2 further supports the pro-apoptotic potential of the composite, as reduced Bcl-2 levels lead to increased mitochondrial outer membrane permeability and promotion of the intrinsic apoptotic pathway. The extent of Bcl-2 downregulation was greater in the MnFe₂O₄/GO-treated group than in cells treated with either MnFe₂O₄ NPs or GO alone, emphasizing the superior efficacy of the composite. The enhanced performance exhibited by MnFe₂O₄/GO NPs arises from the interplay between the unique characteristics of the magnetic NPs and the distinctive properties of graphene oxide. MnFe₂O₄ NPs enhance cellular uptake due to their magnetic properties, while GO provides a large surface area and functional groups that facilitate cellular interactions and delivery. The enhanced capability of the composite to regulate the expression of genes associated with apoptosis, surpassing the effectiveness of its separate constituents, highlights its promise as a potent anticancer therapeutic. The exposure of HeLa cells to MnFe₂O₄/GO NPs results in a substantial upregulation of genes associated with apoptosis, specifically BAX and Caspase-3, while concurrently downregulating the expression of the anti-apoptotic gene Bcl-2. This indicates the NPs’ potent capability to enhance programmed cell death. These results underscore the potential of MnFe₂O₄/GO NPs as an innovative approach for cervical cancer therapy, primarily by modulating apoptotic signaling pathways [50,51,52].
Relative gene expression levels of apoptosis-related genes in HeLa cells treated with GO, MnFe₂O₄ nanoparticles (MnFe₂O₄ NPs), and MnFe₂O₄/GO composite. (a) BAX (pro-apoptotic), (b) Caspase-3 (pro-apoptotic), and (c) Bcl-2 (anti-apoptotic) gene expression levels are presented as log2 fold changes relative to untreated cells
Although the present study focused on evaluating the effects of MnFe₂O₄/GO nanocomposites in a two-dimensional (2D) monolayer model of HeLa cells, which is commonly used for preliminary anticancer screening, it is acknowledged that such models do not fully recapitulate the complexity of in vivo tumor microenvironments. The gene expression data, demonstrating significant upregulation of pro-apoptotic markers (BAX, Caspase-3) and suppression of the anti-apoptotic gene Bcl-2, provide mechanistic insight into the therapeutic potential of the composite. These early findings justify the composite’s progression to more advanced models. While three-dimensional (3D) spheroid cultures or animal studies were not included in the current work due to the study’s focus on synthesis and mechanistic exploration, future investigations will incorporate such models to better assess tumor penetration, pharmacokinetics, and therapeutic efficacy under more physiologically relevant conditions.