Online COVID and reproductive health survey
The study, titled “The COVID-19 Pandemic and Women’s Reproductive Health” received a favourable ethical opinion from the Oxford University School of Anthropology and Museum Ethnography Departmental Research Ethics Committee [SME_C1A_20_029]. The survey was designed to evaluate whether and how the COVID-19 pandemic influenced menstrual health. We incorporated feedback from women suffering from long COVID via long COVID Support (https://www.longcovid.org/). An online survey was launched on March 8, 2021, and was hosted on the Qualtrics platform (www.qualtrics.com). All survey responses were anonymized using randomly generated IDs. This allowed the collection of retrospective and self-reported data on menstrual cycles, behaviour, life circumstances and health before and during the pandemic, as well as COVID-19 disease and vaccination history. The survey included a maximum of 105 questions depending on individual circumstances and took an average of 24 minutes to complete. After consent, 61% of eligible participants answered all questions (on average, participants completed 80% of the questionnaire). To minimise survey fatigue, progress could be saved for up to 14 days to allow participants to resume later. The survey ran from 08/03/21 to 01/06/21 and was closed when there had been no new entries for a week.
Participant eligibility criteria included age >18, having ever menstruated, currently living in the UK, and giving informed consent to the use of their data. The survey was written in English and disseminated through a Facebook advertising campaign targeting all menstruators in the UK, and included images of women of diverse ethnicities, ages, and abilities, as well as images of breastfeeding and pregnant women. The title of the survey was kept general (“women’s reproductive health and the COVID pandemic”) so as not to oversample individuals with a specific interest in menstrual cycles and COVID infection or vaccination. We fine-tuned the ad targeting (to the extent that Facebook allows) throughout the campaign to ensure an even geographical and socioeconomic spread. We also used a stratified sampling strategy to ensure that subgroups of the UK population in terms of age, income and ethnicity were represented in the final sample. In total, 695,543 people viewed the survey ad on their Facebook page, and 26,710 with the eligible criteria gave consent and completed it (there were no duplicates), leading to a 3.8% response rate. In this sample, participants were aged 18–45, 95% identified as being of White ethnicity and 99% identified as women.
Menstrual parameters
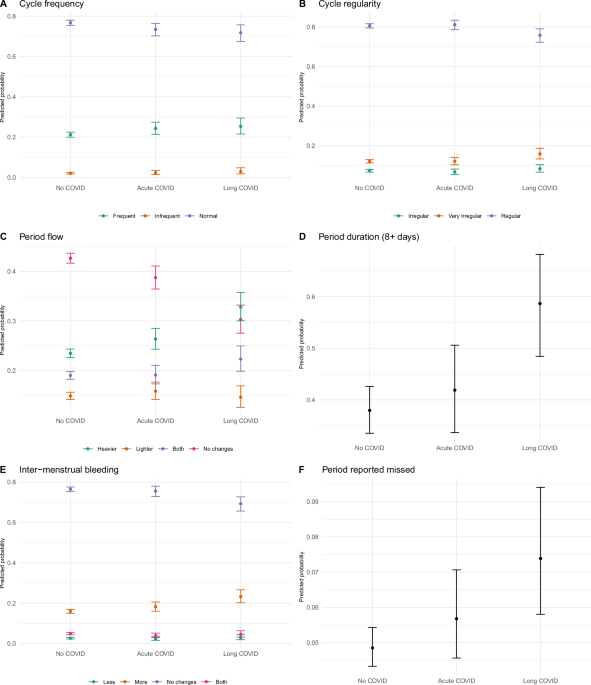
We operationalized our outcome variables to approximate the FIGO classification system for normal and abnormal uterine bleeding in relation to five parameters: frequency, regularity, duration, volume, and inter-menstrual bleeding (FIGO System 1)1.
Frequency
In the latter part of the survey, participants were asked, “Over the last year, how many days long, on average, was your cycle (between the start of one bleed and the start of the next bleed)?”. Based on the number of days reported, we created a variable with 3 possible outcomes (Normal [24–38 days], Frequent [<24 days], Infrequent [>38 days], based on FIGO definitions).
Participants were also asked “Over the last year, have your periods stopped?” and “Over the last year, did you miss your periods at least once?” Although “stop” and “miss” were not defined, concerns over “missing periods” were being reported on social media and thus this variable was meant to capture people’s perception of their cycles from which we created a binary variable (perception of ‘missing’ or ‘stopped’ periods (0/1)).
Regularity
Participants were asked, “Over the last year, how irregular were the length of your menstrual cycles on average?”. We created a variable with 3 possible outcomes (Normal[<2 days; 2–5 days; 5–10 days], Somewhat irregular [10–20 days], Very irregular [>20 days]).
Duration
Participants were asked, “Over the last year, have you noticed any changes in the length of your menstrual cycle? Days of bleeding (Period length)” We created a binary variable with two possible outcomes (Normal ≤8 days; Prolonged >8 + days]).
Volume
“Over the last year, have you noticed any changes in your periods?” There were four possible outcomes (“Heavier”, “Lighter”, “No Changes” and “Heavier and Lighter”).
Intermenstrual bleeding
Over the last year, have you noticed any changes in spotting mid-cycle? There were four possible outcomes (“No changes”, “More”, “Sometimes”, “Sometimes less and sometimes more”.
Exposures
The dataset included socio-demographic variables (age, income, education, gender, ethnic group, marital status, parity), standard proxies for health (BMI, smoking status, physical activity, regular use of vitamins/supplements, regular use of medicine) and reproductive variables indicative of menstrual health before the pandemic (age at menarche, cycle length, period length, cycle irregularity, heavy bleeding, exogenous hormone use and a formal diagnosis of conditions known to affect menstruation, e.g., Endometriosis, Polycystic ovary syndrome, HIV/AIDS, underactive thyroid, overactive thyroid, uterine polyps, uterine fibroids, eating disorders, interstitial cystitis, other), as well as vaccine-related, COVID and pandemic-related variables. COVID-19 disease was operationalized based on whether people thought they had had COVID, as widespread testing had not been available in the UK in the early months of the pandemic that fell within the survey period, leading to three categories: No COVID (no tests or negative tests), acute COVID (symptoms lasting less than 28 days) and long Covid (symptoms lasting more than 28 days; we only included people who had symptoms at least a month before taking up the survey). Exogenous hormone use was categorised as progestogen-only (hormonal coil or IUS, implant, injectable, progestogen-only pill), combined estrogen and progestin (the pill, the patch, vaginal ring), copper IUD, sterilization, none (fertility awareness, condom, female condom, diaphragm) and other (e.g., oral non-contraceptive progestins).
Statistical analysis
R version 4.4.2 (2024-10-31) was used for statistical analysis. We restricted all analyses to pre-menopausal individuals living in the UK who had a period in the 12 months preceding the survey and who were not pregnant or breastfeeding. Further, we only included individuals who knew their COVID-19 disease and vaccination history at the time of the survey. We reported prevalence ratios and relative risk ratios in the text and plotted predicted probabilities from adjusted models to represent absolute effects adjusted for confounders. Our main exposure variable described participants’ self-reported COVID-19 disease history and had 3 levels1 No COVID2; Acute COVID and3 Long COVID. Our referent group was “No COVID”. We used multinomial models when the outcome variables were nominal (two or more categories with no intrinsic order) and log-binomial regressions when the outcome was dichotomous. To evaluate changes between menstrual cycle characteristics, we adjusted all models for menstrual symptom parameters before the pandemic, and included age, BMI, hormonal contraceptive use and presence of diagnosed reproductive disease at baseline as confounders as per hypothesised directed acyclic graphs (https://github.com/ataquette/Long-COVID-Mens). Estimates and confidence intervals on the log-odds scale were converted to relative risk or risk ratios (multinomial models), and those on the log-probability scale (log-binomial models) were converted to prevalence ratios for reporting in tables and figures. To investigate if any associations between our exposure variable and menstrual cycle changes were influenced by confounders, we compared models with and without interaction effects using the Akaike Information Criterion (AIC). We reported variables significant at the false discovery rate (FDR) threshold (FDR-corrected p < 0.05)69.
Missing data
The analysis of complete cases only by dropping missing cases can introduce bias and lead to a substantial reduction of statistical power70, especially if it is plausible that the data are not missing at random or not completely at random. To handle missing data, we used a multiple imputation approach using the R package ‘missRanger’, which combines random forest imputation with predictive mean matching. Prior to all analyses, we imputed 5 datasets, with a maximum of 10 iterations specified for each imputation. Each imputation was also weighted by the degree of missing data for each participant, such that the contribution of data from participants with higher proportions of missingness was weighted down in the imputation. We set the maximum number of trees for the random forest to 200, but left all other random forest hyperparameters at their default. The average out-of-bag error rate for multiple imputation across all imputed datasets was 0.08 (range: 0–0.77). Parameter estimates for all five datasets were pooled to provide more accurate estimates.
Prospective long COVID symptoms study across the menstrual cycle
Participants were recruited on social media via UK long COVID support groups, X and Instagram. All participants provided informed consent prior to taking part in the study and for their anonymized data to be transferred from the Balance App to the French National Centre for Scientific Research (CNRS) for analysis. The study was reviewed by CNRS and received GDPR regulation approval (DPD 2022/10). Recruitment occurred from 01/02/2022 until 13/04/2022, after which no new entries were recorded for at least 2 weeks.
First, participants completed an electronic consent followed by baseline questionnaire at recruitment to record data on socio-demographics (age, gender, residence, height and weight, education, working hours, number of deliveries, caring responsibilities, hormonal contraceptive use, ethnic group, smoking, medication, date of last period, vaccination and covid disease histories, presence and severity of symptoms of long COVID).
Second, participants received daily email links through a reproductive health app (https://www.balance-menopause.com/) to complete a daily survey to record their COVID symptoms and severity over 2 months. The daily questionnaire asked about menstrual volume, with the options of ‘no bleeding’, ‘spotting’, ‘light bleeding’, ‘moderate bleeding’ or ‘heavy bleeding’ before asking participants to rate how they felt each day on a scale of 0–100 (100 being how they felt pre COVID). They were then asked to rate how 29 common long COVID symptoms were affecting them on that day, 0: not at all, 1: a little, 2: quite a bit, 3: a lot, 4: extremely. These symptoms were chosen because previous research suggested that they were experienced by at least 40% of those with long COVID28 and included brain fog/cognitive dysfunction, memory impairment, speech and language symptoms (e.g., difficulty finding words), sensorimotor symptoms (e.g., tingling/pins and needles), dizziness and balance issues, change in smell and taste, insomnia (unable to sleep), headache, disturbed sleep, fatigue, post-exertional malaise (exhaustion after exercise/effort), chills/flushing/sweats, elevated temperature (between 37 C and 38 C or 98.6 F and 100.4 F), heart palpitations (extra awareness of heart beat or irregular heart beat), tachycardia (heart beating too fast), pain/burning in the chest, chest tightness, muscle aches, joint pain, sore throat, vision symptoms (e.g., blurred vision, flashing), tinnitus (ringing in the ears), shortness of breath, dry cough, breathing difficulty, diarrhoea, loss of appetite, nausea, abdominal pain (excluding period pains).
We considered the start of a cycle to be a day marked by spotting or bleeding, where the previous day had no bleeding or spotting, and the following day also showed bleeding or spotting. We examined three outcomes (i) the number of daily COVID-19 symptoms experienced, which corresponds to the number of symptoms experienced each day with a severity score >0 (ii); the presence of each COVID-19 symptom and (iii) the severity of each COVID-19 symptom. Our main exposure was the phase of the menstrual cycle, classified into 3 levels: (i) the perimenstrual phase, which includes the 2 days before menstruation and all subsequent days of menstruation; (ii) the proliferative phase, from the first day without bleeding until 15 days before the next cycle; and (iii) the secretory phase, from 14 days to 3 days before the next cycle.
To analyse the number of daily symptoms, we first ran non-parametric Friedman tests for paired data to compare the median number of daily symptoms across the three distinct phases of the menstrual cycle, in a sample of individuals who contributed data to all three phases (n = 32). We then included all data and ran a minimally age-adjusted mixed Poisson regression routine using the package lmer4 to account for an unbalanced dataset, as well as repeated daily measures of symptoms across individuals and phases. Age was transformed into a category variable following the median, (
To analyse the presence of each symptom on a given day, we ran a series of multilevel binomial regressions adjusted for age. To analyse the severity of each symptom experienced, we conducted a cumulativelink mixed model for ordinal outcomes. It was not possible to adjust for age or other covariates due to the limited number of points for some symptoms. Estimates and confidence intervals on the log scale were converted to rate ratios (Poisson model) and those on the log-probability scale (log-binomial models) were converted to risk ratios for reporting in tables and figures. We reported variables significant at the FDR threshold (FDR-corrected p < 0.05)69.
Biological sample collection from those with long COVID and controls
Blood serum and an optional endometrial biopsy were collected from 10 participants with longer term symptoms of COVID (present for more than 4 weeks, based on consensus definitions at the time of recruitment) after informed consent and with a favourable ethical opinion from East Midlands-Leicester South Research Ethics Committee (21/EM/0166), Fig. 1. Participants responded to a study poster on social media and disseminated via Scottish online support groups for those with long COVID. Participants were 33–45 years old, had regular menstrual cycles (24–38 days) and reported ethnicity as White (English/Scottish/Welsh/Northern Irish, n = 8) or mixed/multiple ethnic group (n = 2) (Table 2). Four participants had previously had positive PCR tests for SARS-CoV, and the remaining six had symptoms confirmed to be consistent with acute infection by a healthcare professional. All participants received COVID vaccinations (from various manufacturers, n = 7 had received three doses, n = 2 had received 2 doses and n = 1 had received 1 dose). No participants were using exogenous hormones or an intrauterine device during the study or in the preceding 2 months. No participants were taking anti-coagulant medications. Those with known fibroids >3 cm, reproductive tract cancer, currently breastfeeding or using oral steroids were excluded. On average, participants had experienced long COVID symptoms for ~15 months at the time of study consent. Participants were asked which symptom was most troubling, and this was reported as fatigue in 5/10 participants.
Participants attended NHS Lothian, Scotland, UK, between November 2021 and April 2023 on three occasions; during menstruation, within a week of menstruation cessation and 7 days prior to the typical cycle length, aiming for the mid-secretory phase. Serum samples were collected and confirmed as menstrual (n = 10), proliferative (n = 10), and secretory (n = 7) (Fig. 2B), based on cycle length and date of last menstrual period. Two samples timed to capture the secretory phase were excluded due to likely anovulation on analysis of serum estradiol and progesterone on the day of the biopsy. The rates of anovulation in this study were similar to our previous studies prior to the COVID pandemic. Time of sample collection was between 0900 and 1200 for 17 samples (63%), between 1200 and 1700 for nine (33%), and not recorded for one (4%).
Endometrial biopsies were collected using an endometrial sampler in the outpatient department, with stage of cycle confirmed by consistency across three parameters of (i) last menstrual period, (ii) serum estradiol and progesterone at time of biopsy and (iii) histological grading by a consultant pathologist71. Samples were confirmed as menstrual (n = 5), proliferative (n = 7) and secretory (n = 4) (Fig. 1). Regarding timing of endometrial samples, seven were collected between 0900 and 1200 (44%) and nine between 1200 and 1700 (56%). Immediately after collection, tissue was divided and placed in RNAlater™ stabilization solution [Invitrogen by ThermoFisher Scientific, Leicestershire, UK, AM7020] and stored at −80 °C for RNA extraction and neutral buffered formalin prior to paraffin wax embedding.
Control blood serum samples (n = 54) and endometrial samples (n = 32) were selected from the Female Reproductive Tract Tissue Resource (20/ES/0119), limited to those collected from women (n = 40) prior to November 2019 (pre-COVID-19 pandemic) and matched for age and parity. The time of serum collection from control participants was between 0900 and 1200 in 26 (48%) and between 1200 and 1700 in 19 (35%) samples, with no time recorded for nine samples (17%). Endometrial samples were collected between 0900 and 1200 for n = 16 (50%) and between 1200 and 1700 for n = 15 (47%), with no time recorded for n = 1 (3%) endometrial sample.
An additional group (n = 10) of women who had fully recovered from previous SARS-CoV-2 infection (confirmed by lateral flow or PCR test) was recruited. Serum blood samples were provided on three occasions across the menstrual cycle after informed consent and a favourable ethical opinion (24-EMREC-015). Participants responded to a study poster disseminated via university mailing lists and were 24–45 years old, had regular menstrual cycles and reported ethnicity as white (n = 9) or mixed (n = 1) (Table 2). All participants received COVID vaccinations from various manufacturers, n = 1 had received 5 doses, n = 1 had received 4 doses, n = 4 had received 3 doses, n = 2 had received 2 doses, and n = 1 had received 1 dose. Similar to long COVID recruitment, no participants were using exogenous hormones during the study or in the preceding 2 months, and those with reproductive tract cancer, currently pregnant or breastfeeding or using oral steroids were excluded. Of note, it was not possible to match this acute COVID group to the long COVID group for age, BMI or parity. Serum samples were confirmed as menstrual (n = 10), proliferative (n = 10), and secretory (n = 10) (Fig. 2B), based on cycle length, date of last menstrual period and estradiol and progesterone levels at time of sample provision. The time of serum collection was between 0900 and 1200 in 15 (50%) samples and between 1200 and 1700 in 15 (50%) samples.
Immunoassay
Blood serum samples were measured for ovarian sex hormone (estradiol and progesterone) levels using Roche Diagnostic’s (Rotkreuz, Switzerland) automated Electrochemiluminescence Immunoassay Elecsys system on a Cobas e411 analyser to stage serum and endometrial samples (Table 3). The assay detection ranges were; 18.4–11010 pmol/L for estradiol (Roche, 06656021190), 0.159–191 nmol/L for progesterone (Roche, 07092539190). The average inter-assay CVs were 7.1% and 13%, respectively.
Table 3 Summary of serum hormone levels for control and long COVID groups at three stages of the menstrual cycleLiquid chromatography tandem mass spectrometry (LC-MS/MS)Sample preparation
Blood serum samples (200 µL) were enriched with isotopically labelled internal standards and transferred to a Supported Liquid Extraction 96-well plate (ISOLUTE, SLE + 400, Biotage, Uppsala, Sweden) along with a calibration curve of multiple steroid standards (0.0025–100 ng). Samples and standards were eluted with 98:2 (v/v) dichloromethane/isopropanol, the resulting extracts reduced to dryness and then reconstituted in 100 µL 70:30 (v/v) water/methanol.
Endometrial tissue (5–10 mg) from a subset of participants with sufficient tissue (n = 17 controls, n = 14 Long COVID) was placed in 2 mL microfuge tubes containing (2.8 mm) ceramic beads (ThermoFisher Scientific, Leicestershire, UK), suspended in 500 µL 99.9:0.1% v/v acetonitrile/formic acid, enriched with internal standards (20 ng of Working Internal Standard, WIS). Samples were homogenised on a cryo-cooled Bead Mill Homogeniser (Omni International, UK), centrifuged and transferred to an ISOLUTE phospholipid depletion 96-well plate (PLD+, Biotage) alongside a calibration curve of standards and extracted. The final sample numbers for endometrial tissue LC-MS/MS analysis were lower than those in Fig. 2B due to endometrial tissue sample depletion. Menstrual phase: long COVID n = 3, control n = 5. Proliferative phase: long COVID n = 7, control n = 6. Secretory phase, long COVID n = 4, control n = 6.
Steroid profiling by LC-MS/MS
Serum and tissue steroid extracts were profiled on an Acquity I-Class UPLC system and QTrap 6500+ mass spectrometer (AB Sciex, UK) system adapted from a previously described LC-MS/MS method72,73. All steroid analytical standards used were certified reference material solutions as provided by Sigma-Aldrich/Cerilliant at either 1 mg/mL or 100 mg/mL in methanol or acetonitrile. 20 µL of each calibration standard and sample were sequentially injected onto a Kinetex C18 (150 × 2.1 mm, 2.6 µm) column with a mobile phase system of A-water and 0.05 mM ammonium fluoride, B-methanol and 0.05 mM ammonium fluoride, starting at 50% B, rising to 75% B over 9 minutes, then 100% B by 12 minutes and returning to 50% B at 16 minutes. The eluate from the LC column was transferred to a QTrap 6500+ mass spectrometer instrument for mass analysis by multiple reaction monitoring. The peak area of the steroid, divided by the internal standard, was used to generate calibration curves. Limits of Quantitation (LOQ) for each steroid in serum were 0.05 ng/mL for cortisol, 0.05 ng/mL for cortisone, 0.05 ng/mL for testosterone, 0.01 ng/mL for 5α-dihydrotestosterone, 0.125 ng/mL for progesterone, 0.25 ng/mL for 17α-hydroxyprogesterone, 0.0125 ng/mL for 17beta-estradiol and 0.03 ng/mL for estrone. LOQ for steroids in tissue in ng/g; 0.005 ng for cortisol, testosterone and cortisone, 0.0125 ng for 5α-dihydrotestosterone and 17β-estradiol and 0.125 ng for progesterone and 0.0063 ng for estrone.
Multiplex automated ELISA
Blood serum samples were measured for a panel of inflammatory cytokines (IFNG, IL-2, IL-6, IL-8, IL-10 and TNF) using the Ella automated enzyme-linked immunosorbent assay (ELISA) platform (Bio-Techne, Minneapolis, USA), which utilises microfluidic channels to measure each sample in triplicate. Samples were diluted 1:2 and loaded onto SimplePlex Cartridge’s (ST01E-PS-005711, Lot# 31895 and 34601), run on an Ella 600-100 system and analysed using SimplePlex software (all Bio-Techne) as per manufacturer’s instructions. The assay ranges were; IFNG 0.17–2000 pg/mL, IL-6 0.28–2652 pg/mlL IL-8 0.19–1804 pg/mL, IL-10 0.58–2212 pg/mL, IL-2 0.54–2,050 pg/mL and TNF 0.7–4000 pg/mL. The serum samples that were lower than the SimplePlex assay lot# defined lower limits of quantification (LLOQ), were assigned the lower detection limit value for each assay. IL-2 values were below the limit of detection in all but one serum sample; therefore, these data were excluded.
AMH ELISA
AMH levels were measured in proliferative phase blood serum samples using Ansh Labs picoAMH ELISA (Texas, USA, AL-124-i, Lot# 101823) as per manufacturer’s instructions. The blood serum samples were measured in duplicate, along with six calibrators and two controls supplied with the kit. The results were read on a TECAN LT-4500 Microplate Absorbance Reader (LabTech, Switzerland) and analysed using SoftMaxPro Validation software (Molecular Devices, California, USA). The assay range was 6–980 pg/mL and the intra-assay CV was 4.0% (0.2–9.5%).
Reverse transcription quantitative PCR (RTqPCR)
Total RNA was extracted from endometrial biopsy tissue samples using RNeasy Plus Mini Kits (QIAGEN, Venlo, The Netherlands, 74134) and complementary DNA (cDNA) generated using iScript cDNA Synthesis (Bio-Rad Laboratories, California, USA, 1708890). qPCR assays were designed using the National Centre for Biotechnology Information (NCBI, Maryland, USA) Reference Sequence Database (RefSeq) to confirm target gene identity and Roche Applied Science’s (Penzberg, Germany) Universal Probe Library (UPL) Assay Design Centre to select primer pairs and probes (Table S10). TaqMan qPCR was carried out using TaqPath ProAmp Mastermix (ThermoFisher, A30866) on an Applied Biosystems (ABI) QuantStudio 5 real-time PCR system (ThermoFisher). Samples and controls were analysed in triplicate with QuantStudio Design and Assay software using the comparative threshold method. One biological replicate was removed from the assessment of INFG in endometrial tissue due to Ct values being above the threshold for detection (undetectable). Messenger RNA (mRNA) transcripts were normalized relative to the geomean of two appropriate reference genes, ATP5B and SHDA, as determined with the geNorm algorithm (PrimerDesign, Eastleigh, UK). Samples were quantified relative to a positive endometrial control (mixed menstrual stage) sample.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded endometrial biopsy tissue samples were cut to 5-µm sections, dewaxed in Histo-Clear (National Diagnostics, Atlanta, USA, NAT1334) and rehydrated through graded alcohol (100, 95, 80, and 70%) and water. IHC was carried out on a Leica Microsystems (Milton Keynes, UK) BOND-III staining robot using the BOND Polymer Refine Detection system (Leica Microsystems, DS9800), a modified 3,3’-Diaminobenzidine tetrahydrochloride hydrate chromogen and a hematoxylin counterstain protocol, with standard BOND reagents. The specific primary antibody and Refine protocols used are detailed in Table S11. Tissue sections were then dehydrated through graded alcohol (70, 80, 95 and 100%), cleared in Histo-clear and mounted with Pertex (CellPath, Newton, UK, SEA-0100-00A). Slides were digitally recorded with the Axioscan Z1 Brightfield Microscope slide-scanner at ×20 resolution and manipulated using Zen Blue Software (both Zeiss, Carl Zeiss Microscopy, Cambridge UK). All good quality menstrual endometrial images are shown in panels, with 3 representative samples selected for proliferative and secretory phases.
IHC quantification was performed using QuPath Version 0.5.0, with the investigator blind to experimental group74. Positive cell detection parameters were used to identify and classify stained nucleated cells in endometrial tissue. Detection thresholds were optimised for each antibody using representative training images. The immune cell marker (CD68 and LL37) positive staining threshold was 0.3, with % of positive nucleated cells reported. Sex receptor (AR and PR) staining threshold was 0.1 and intensities were 0.1–0.4 (weak = 1), 0.4–0.7 (medium = 2) and >0.7 (strong = 3). Histoscore was determined by the sum of % positive nucleated cells after multiplication by staining intensity.
Statistical analysis
Analysis was carried out using GraphPad Prism 10.4.1. For comparison of multiple datasets with two grouping variables (i.e., long COVID versus controls and stage of menstrual cycle), a two-way analysis of variance was used, with Tukey’s multiple comparisons test. A value of P < 0.05 was considered significant. Subset analysis of paired samples from the same participant (e.g., serum samples from the secretory and menstrual phases) was analysed using Wilcoxon and paired t-tests, depending on data distribution.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.