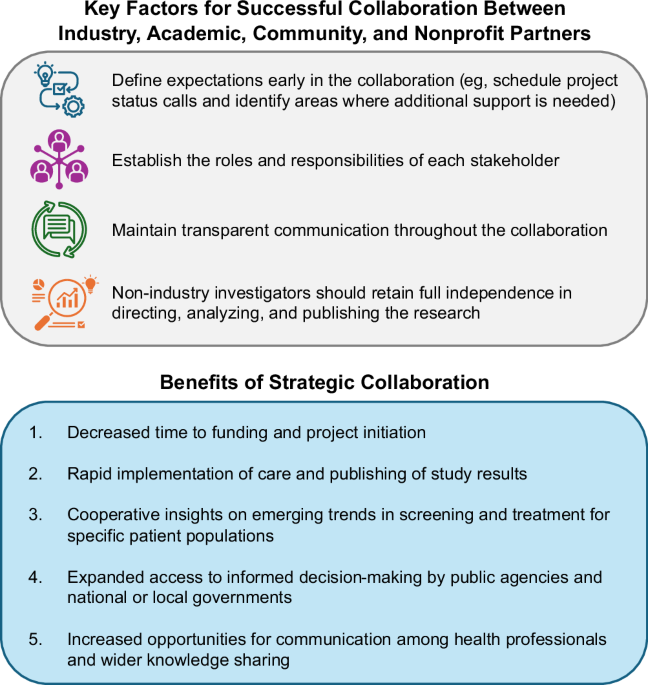
Simplified care cascades with easier treatment access are needed to ensure optimal treatment uptake with minimal patient loss to follow-up10. The primary benefit of programs such as LEGA-C is their ability to rapidly implement sustainable screening, linkage to care, treatment, and monitoring for people with HCV, wherever they are located (Table 1). In the Balearic Islands, Spain, PWUD who had an active HCV infection and participated in the Hepatitis C Free Balears study were offered access to a decentralized treatment process11. Participants were telematically prescribed direct-acting antivirals (DAAs) by a hepatologist, with medication delivered either from a hospital pharmacy or a local addiction center11. This decentralized health care model demonstrated a high acceptance rate of the intervention among a patient population that has been historically stigmatized11. The model was also implemented across addiction centers on the main islands11. A similar LEGA-C—funded outreach program for PWUD in Italy was implemented at 15 regional Services for Dependence (SERDs) and also provided a shuttle service, which was continued by some local community SERDs after study completion12. Both studies highlight the successful impact of decentralized care models for marginalized people with HCV infection who often lack access to medical care, with both programs achieving a sustained virologic response (SVR) rate ≥90% at ≥12 weeks posttreatment11,12. These studies also demonstrate how LEGA-C funding enabled academic researchers to develop a care model for an often-marginalized population for which care was initially controversial, but is now included in HCV treatment guidelines7,13. By extension, these studies offer a framework for similar future partnerships focused on global HCV elimination (Fig. 2).
Fig. 2: Summary of SVR rates observed across LEGA-C—funded studies on each continent.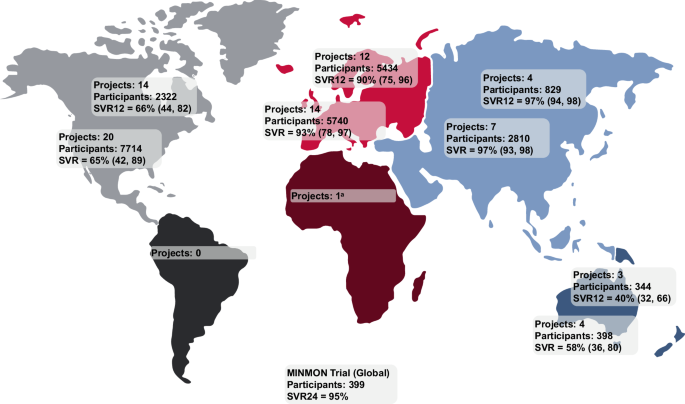
Data are shown as the total number of participants monitored for SVR and median (Q1, Q3) SVR12 or SVR across all projects per continent. The time point at which SVR is reported varies by project, with some studies using SVR12 as the primary endpoint, while others report SVR24 or SVR. aSVR data not reported. LEGA-C local elimination programs leading to global action in HCV, MINMON minimal monitoring, Q quartile, SVR sustained virologic response, SVR12 SVR at posttreatment week 12, SVR24 SVR at posttreatment week 24.
Table 1 Selected studies to represent industry and non-industry partners that focus on programs for HCV elimination
In LMICs, LEGA-C funding has supported several HCV microelimination operational initiatives, such as the 1-year pilot Uzbekistan Hepatitis Elimination Project that included simultaneous rapid testing for HCV and hepatitis B virus (HBV), training for primary care physicians to treat patients with HCV or HBV without advanced liver disease, and the provision of free or below-market-price treatment14. This pilot project led to a subsequent 2-year project and culminated in a presidential decree establishing government funding and resources for HBV/HCV screening, HCV treatment, and HBV vaccination for health care workers14. In Pakistan, an HCV microelimination study targeted an endemic district of 40,000 individuals, with field teams visiting households to diagnose and treat participants with HCV infection via monthly follow-up visits15. Important findings were the high rates of treatment completion (87%, 1527 of 1758 individuals who had active viremia and started treatment) and SVR (91%, 847 of 933 individuals tested 12 weeks posttreatment)15. This study demonstrates the feasibility of simplified, low-cost, community-based operational programs for HCV microelimination in highly endemic regions of LMICs15.
Many of the LEGA-C studies generated evidence that has informed policy decisions related to HCV screening, diagnosis, and treatment guidelines. The ERASE-C outreach campaign in Taiwan focused on screening, diagnosis, and on-site group treatment of HCV among patients with uremia on hemodialysis16. On-site treatment led to successful HCV microelimination in hemodialysis centers and provided evidence supporting the safety, efficacy, and effectiveness of DAAs16, ultimately leading to updated practice guidelines16,17. Another outreach campaign for an HCV-hyperendemic community in Taiwan demonstrated the effectiveness of decentralized, on-site screening and treatment in improving HCV care cascade outcomes, even amidst the challenges posed by the COVID-19 pandemic18. Emphasizing the decentralization of care to lower-level facilities and task-sharing with non-specialist providers—core principles of international societies—has enhanced the impact on HCV elimination by improving access to care19,20,21.
Another advancement to guidelines came through the minimal monitoring (MINMON) study, a collaborative global effort with the AIDS Clinical Trials Group (A5360). This study demonstrated that minimal monitoring and reduced time to treatment initiation have the potential to accelerate HCV elimination22. The interventional study of treatment with sofosbuvir and velpatasvir for 12 weeks applied a 4-component approach: (1) no pretreatment genotyping, (2) dispensing the entire treatment course at study entry, (3) no scheduled visits or laboratory monitoring, and (4) two points of remote contact (one at week 4 for adherence, and another at week 22 to schedule an assessment of SVR at week 24)22. The results highlighted that with DAAs, the full HCV treatment course can be delivered and completed safely without on-treatment monitoring, and this approach is now recommended in both the American Association for the Study of the Liver and EASL guidelines7,22,23.