Dublin, Oct. 03, 2025 (GLOBE NEWSWIRE) — The “U.S. Cell And Gene Therapy Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, II, III, IV), By Indication (Oncology, Cardiology, CNS, Musculoskeletal, Infectious Diseases), And Segment Forecasts, 2025 – 2033” report has been added to ResearchAndMarkets.com’s offering.
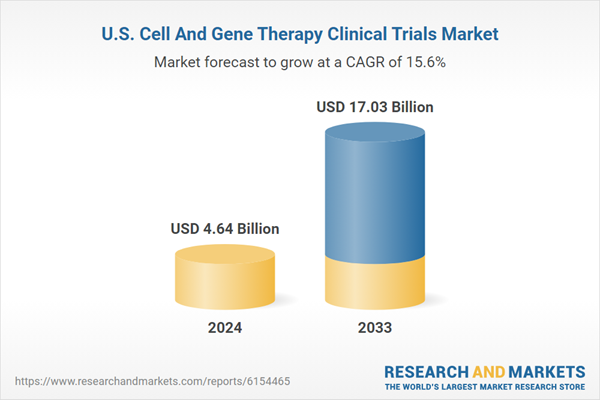
The U.S. cell and gene therapy clinical trials market size was estimated at USD 4.64 billion in 2024 and is projected to reach USD 17.03 billion by 2033, growing at a CAGR of 15.60% from 2025 to 2033.
The market is driven by an increase in R&D funding, increasing disease burden in oncology, rare genetic disorders, and neurodegenerative conditions, growing interest in cell and gene therapies for cancer treatment, and a favorable regulatory environment.
Besides, the surge in pipeline candidates with over 2,000 CGT assets is currently in development at various preclinical and clinical stages. It has benefited from substantial R&D funding provided by biopharma companies, startups, and government entities. In addition, regulatory advancements, including the FDA’s accelerated approval pathways, RMAT designations, and INTERACT meetings, support the need for cell and gene therapy initiation and development of trials.
Moreover, increased investments and M&A activities from large pharmaceutical companies are propelling the expansion of CGT trials, particularly in autologous CAR-T therapies, AAV-based gene therapies, and allogeneic platforms. Service providers and CDMOs are enhancing their CGT-specific capabilities, such as GMP vector production, viral delivery systems, and decentralized trial logistics.
U.S. Cell And Gene Therapy Clinical Trials Market Report Segmentation
This report forecasts revenue growth at country level and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, the analyst has segmented the U.S. cell and gene therapy clinical trials market report based on phase and indication.
Phase Outlook (Revenue, USD Million, 2021 – 2033)
Phase IPhase IIPhase IIIPhase IV
Indication Outlook (Revenue, USD Million, 2021 – 2033)
OncologyCardiologyCNSMusculoskeletalInfectious diseasesDermatologyEndocrine, metabolic, geneticImmunology & inflammationOphthalmologyHematologyGastroenterologyOthers
This report addresses:
Market intelligence to enable effective decision-makingMarket estimates and forecasts from 2018 to 2030Growth opportunities and trend analysesSegment and regional revenue forecasts for market assessmentCompetition strategy and market share analysisProduct innovation listing for you to stay ahead of the curve
Key Attributes:
Report AttributeDetailsNo. of Pages150Forecast Period2024 – 2033Estimated Market Value (USD) in 2024$4.64 BillionForecasted Market Value (USD) by 2033$17.03 BillionCompound Annual Growth Rate15.6%Regions CoveredUnited States
Key Topics Covered:
Chapter 1. Research Methodology and Scope
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. U.S. Cell and Gene Therapy Clinical Trials Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Growing Interest in Cell and Gene Therapy
3.2.1.2. Adoption of New Technology in Clinical Research
3.2.1.3. Increasing Investments and R&D Funding
3.2.1.4. Rising Prevalence of Rare and Genetic Disorders
3.2.2. Market Restraint Analysis
3.2.2.1. Recruitment Obstacles
3.3. Pricing Model Analysis
3.4. Technology Landscape
3.5. Market Analysis Tools
3.5.1. Porter’s Five Force Analysis
3.5.2. PESTEL by SWOT Analysis
3.5.3. COVID-19 Impact Analysis
Chapter 4. U.S. Cell and Gene Therapy Clinical Trials Market: Phase Estimates & Trend Analysis
4.1. U.S. Cell and Gene Therapy Clinical Trials Market, By Phase: Segment Dashboard
4.2. U.S. Cell and Gene Therapy Clinical Trials Market, By Phase: Movement Analysis
4.3. U.S. Cell and Gene Therapy Clinical Trials Market Estimates & Forecasts, By Phase, 2021 – 2033
4.4. Phase I
4.5. Phase II
4.6. Phase III
4.7. Phase IV
Chapter 5. U.S. Cell and Gene Therapy Clinical Trials Market: Indication Estimates & Trend Analysis
5.1. U.S. Cell and Gene Therapy Clinical Trials Market, By Indication: Segment Dashboard
5.2. U.S. Cell and Gene Therapy Clinical Trials Market, By Indication: Movement Analysis
5.3. U.S. Cell and Gene Therapy Clinical Trials Market Estimates & Forecasts, By Indication, 2021 – 2033
5.4. Oncology
5.5. Cardiology
5.6. CNS
5.7. Musculoskeletal
5.8. Infectious diseases
5.9. Dermatology
5.10. Endocrine, metabolic, genetic
5.11. Immunology & inflammation
5.12. Ophthalmology
5.13. Hematology
5.14. Gastroenterology
5.15. Others
Chapter 6. Competitive Landscape
6.1. Market Participant Categorization
6.2. Company Market Share/Assessment Analysis, 2024
6.3. Company Profiles
IQVIAICON PlcLabCorpCharles River LaboratoriesPAREXEL International Corp.Syneos HealthMedpaceThermo Fisher Scientific, Inc.NovotechVeristat, LLC
For more information about this report visit https://www.researchandmarkets.com/r/6z74za
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world’s leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
U.S. Cell And Gene Therapy Clinical Trials Market


