Patient consent
This study was approved by the Columbia University Irving Medical Center Institutional Review Board, with a waiver of informed consent. This study was approved through the Committee for the Protection of Human Subjects at UTHealth, Houston and did not require an informed consent as it was retrospective.
Data
We conducted a retrospective manual review of electronic medical record data of patients with confirmed mpox from three clinical sites in New York City, New York and Houston, Texas from June 25 to October 17, 2022. The New York City site used a Research Electronic Data Capture (REDCap) database. Patients were defined as confirmed mpox cases if they had positive orthopoxvirus polymerase chain reaction (PCR) testing.
Test assays and testing criteria
Screening for STI co-infections in people with mpox was performed using the Aptima Combo 2 assay for GC and CT (Hologic, Marlborough, Massachusetts) and a reactive plasma reagin (RPR) followed by a confirmatory fluorescent treponemal antibody absorption. Testing for GC and CT was available at urinary, rectal, and pharyngeal sites. Self-testing was performed for rectal and pharyngeal assays, consistent with standard of care. Testing was performed at the discretion of treating clinicians.
Statistical analysisDescriptive statistics
Baseline demographic and clinical characteristics of the cohort were summarized using frequencies and percentages for categorical variables. Characteristics analyzed included age, sex at birth, race and ethnicity, HIV status, pre-exposure prophylaxis (PrEP) usage, presence of genital or anal lesions, and use of tecovirimat. Genital lesions included any lesions on male or female genitalia as well as the anorectal area and groin, suprapubic or pubic areas.
Testing rates
Testing rates for STIs, including GC, CT, and syphilis, were calculated as the proportion of patients tested for each infection among the total cohort. These rates were further stratified by anatomical site (genitourinary, rectal, pharyngeal) and the presence or absence of site-specific symptoms.
Positivity rates
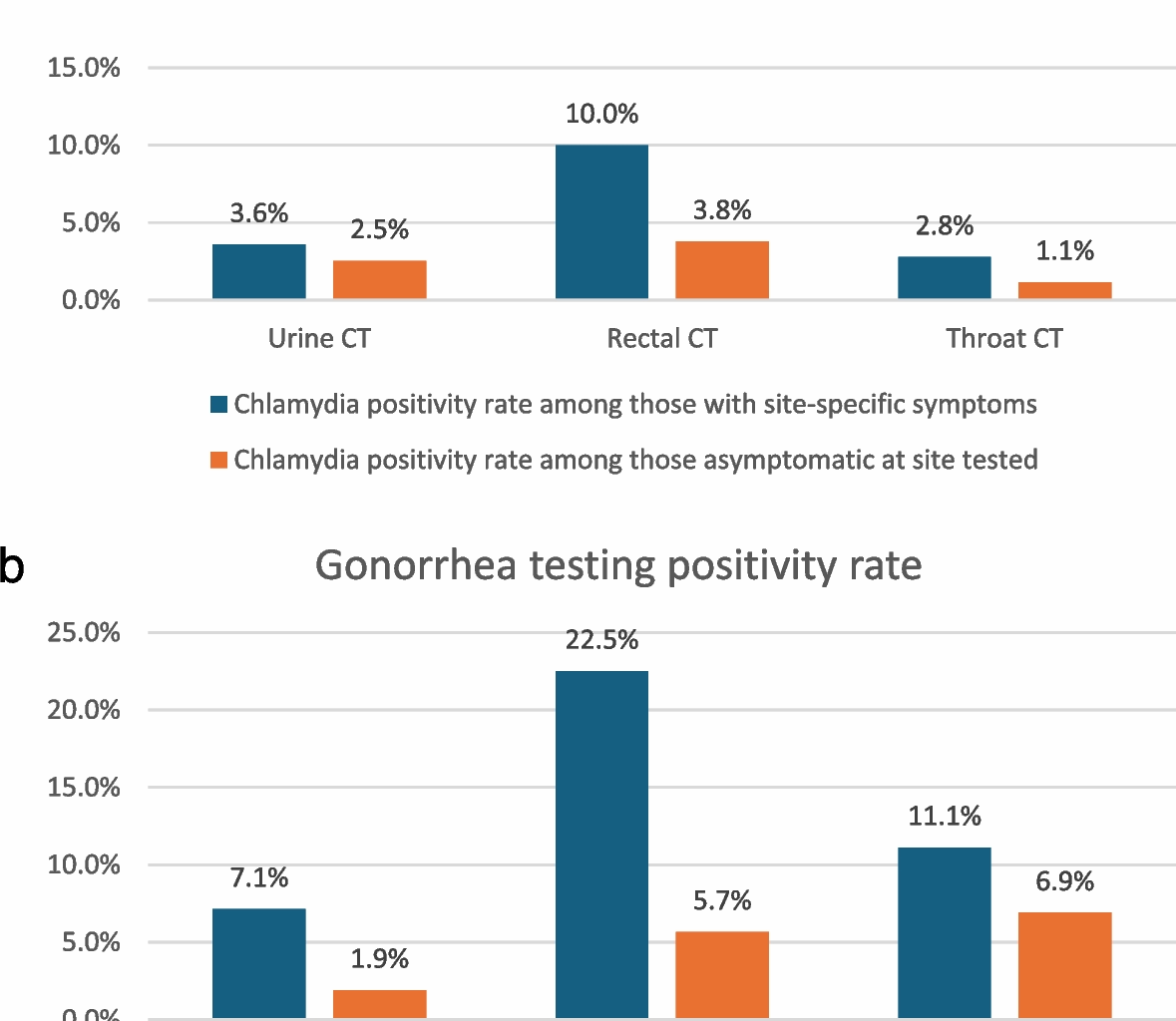
Positivity rates for GC and CT were calculated for each anatomical site, stratified by the presence or absence of patient-reported site-specific symptoms (e.g., sore throat, penile or rectal discharge, rectal pain, discomfort, burning, etc.) that were assessed by manual chart review of each case. Comparisons were made to assess differences in positivity rates between symptomatic and asymptomatic individuals.
Symptom-specific testing and diagnosis
The proportion of patients reporting site-specific symptoms (e.g., rectal, pharyngeal, or urinary symptoms) who underwent corresponding STI testing was calculated. Among those tested, the positivity rates for GCand CT were evaluated.
Missed diagnoses
To estimate the number of missed infections among untested patients, we applied observed positivity rates stratified by symptom status (symptomatic vs. asymptomatic) as proxies for expected prevalence in the untested cohort. For symptomatic individuals, site-specific positivity rates derived from those tested were used to extrapolate the likely number of missed cases among untested patients reporting similar symptoms (e.g., rectal, pharyngeal, or urinary). For asymptomatic individuals, overall positivity rates from asymptomatic patients tested were used as ‘background’ prevalence to estimate the number of missed infections among untested asymptomatic individuals. Calculations were performed separately for GC and CT at each anatomic site (genitourinary, rectal, and pharyngeal), and results were aggregated to quantify potential underdiagnosis across the cohort. This approach assumes that the distribution of infection prevalence in the untested population mirrors that of the tested population with similar symptomatology.
Software
All statistical analyses were performed using R version 4.2.1 and RStudio 2022.02.3 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics version 28 (IBM, Armonk, New York, United States of America) as well as Microsoft Excel (Microsoft, Redmond, Washing, United States of America). Statistical significance was defined as a two-tailed p-value < 0.05.