Coffee and Caffeine Genetics Consortium, Cornelis MC, Byrne EM, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015;20(5):647–56. https://doi.org/10.1038/mp.2014.107.
Roberts A. Caffeine: an evaluation of the safety database. In: Gupta RC, Lall R, Srivastava A, editors. Nutraceuticals. 2nd ed. Cambridge: Academic Press; 2021. p. 501–18. https://doi.org/10.1016/B978-0-12-821038-3.00032-X.
Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110–3. https://doi.org/10.1016/j.jada.2004.10.027.
Martins RS, Rombo DM, Gonçalves-Ribeiro J, et al. Caffeine has a dual influence on NMDA receptor–mediated glutamatergic transmission at the hippocampus. Purinergic Signal. 2020;16(4):503–18. https://doi.org/10.1007/s11302-020-09724-z.
Fredholm BB. Astra Award Lecture: adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76(2):93–101. https://doi.org/10.1111/j.1600-0773.1995.tb00111.x.
Quadra GR, Paranaíba JR, Vilas-Boas J, et al. A global trend of caffeine consumption over time and related-environmental impacts. Environ Pollut. 2020;256:113343. https://doi.org/10.1016/j.envpol.2019.113343.
Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land Psychopharmacol. 2002;164(3):250–61. https://doi.org/10.1007/s00213-002-1217-9.
Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology. 2005;179(4):813–25. https://doi.org/10.1007/s00213-004-2104-3.
Gutiérrez-Hellín J, Varillas-Delgado D. Energy drinks and sports performance, cardiovascular risk, and genetic associations; future prospects. Nutrients. 2021;13(3):715. https://doi.org/10.3390/nu13030715.
Kolb H, Kempf K, Martin S. Health effects of coffee: mechanism unraveled? Nutrients. 2020;12(6):1842. https://doi.org/10.3390/nu12061842.
Kowiański P, Lietzau G, Steliga A, et al. Nicotine-induced CREB and DeltaFosB activity is modified by caffeine in the brain reward system of the rat. J Chem Neuroanat. 2018;88:1–12. https://doi.org/10.1016/j.jchemneu.2017.10.005.
Huang ZL, Zhang Z, Qu WM. Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol. 2014;119:349–71. https://doi.org/10.1016/B978-0-12-801022-8.00014-3.
Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133.
Acquas E, Tanda G, Di Chiara G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2002;27(2):182–93. https://doi.org/10.1016/S0893-133X(02)00290-7.
Lazarus M, Shen HY, Cherasse Y, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci Off J Soc Neurosci. 2011;31(27):10067–75. https://doi.org/10.1523/JNEUROSCI.6730-10.2011.
Manalo RVM, Medina PMB. Caffeine protects dopaminergic neurons from dopamine-induced neurodegeneration via synergistic adenosine-dopamine D2-like receptor interactions in transgenic Caenorhabditis elegans. Front Neurosci. 2018;12:137. https://doi.org/10.3389/fnins.2018.00137.
Singh K, Singh S, Singhal NK, Sharma A, Parmar D, Singh MP. Nicotine- and caffeine-mediated changes in gene expression patterns of MPTP-lesioned mouse striatum: implications in neuroprotection mechanism. Chem Biol Interact. 2010;185(2):81–93. https://doi.org/10.1016/j.cbi.2010.03.015.
O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108(1):18–58. https://doi.org/10.1016/j.pharmthera.2005.06.018.
Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev. 1999;23(4):563–76. https://doi.org/10.1016/S0149-7634(98)00050-5.
Favrod-Coune T, Broers B. Addiction to caffeine and other xanthines. In: El-Guebaly N, Carrà G, Galanter M, Baldacchino AM, editors. Textbook of addiction treatment: international perspectives. Berlin: Springer International Publishing; 2021. p. 215–28. https://doi.org/10.1007/978-3-030-36391-8_16.
Zduńska A, Cegielska J, Zduński S, Domitrz I. Caffeine for headaches: helpful or harmful? A brief review of the literature. Nutrients. 2023;15(14):3170. https://doi.org/10.3390/nu15143170.
Ferré S. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology. 2016;233(10):1963–79. https://doi.org/10.1007/s00213-016-4212-2.
Alasmari F. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J. 2020;28(4):445–51. https://doi.org/10.1016/j.jsps.2020.02.005.
Temple JL, Hostler D, Martin-Gill C, et al. Systematic review and meta-analysis of the effects of caffeine in fatigued shift workers: implications for emergency medical services personnel. Prehosp Emerg Care. 2018;22(sup1):37–46. https://doi.org/10.1080/10903127.2017.1382624.
Jessel CD, Narang A, Zuberi R, Bousman CA. Sleep quality and duration in children that consume caffeine: impact of dose and genetic variation in ADORA2A and CYP1A. Genes. 2023. https://doi.org/10.3390/genes14020289.
Tiffin P, Ashton H, Marsh R, Kamali F. Pharmacokinetic and pharmacodynamic responses to caffeine in poor and normal sleepers. Psychopharmacology. 1995;121(4):494–502. https://doi.org/10.1007/BF02246500.
Yang A, Palmer AA, de Wit H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology. 2010;211(3):245–57. https://doi.org/10.1007/s00213-010-1900-1.
Chwedorowicz R, Łukawski K, Raszewski G, Czuczwar SJ. Caffeine impairs anticonvulsant effects of levetiracetam in the maximal electroshock seizure threshold test in mice. J Basic Clin Physiol Pharmacol. 2023;34(3):357–64. https://doi.org/10.1515/jbcpp-2022-0224.
Saunders B, da Costa LR, de Souza RAS, Barreto G, Marticorena FM. Caffeine and sport. In: Toldrá F, editor. Advances in food and nutrition research, vol. 106. Cambridge: Academic Press; 2023. p. 95–127. https://doi.org/10.1016/bs.afnr.2023.03.002.
Cornelis MC, Kacprowski T, Menni C, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;25(24):5472–82. https://doi.org/10.1093/hmg/ddw334.
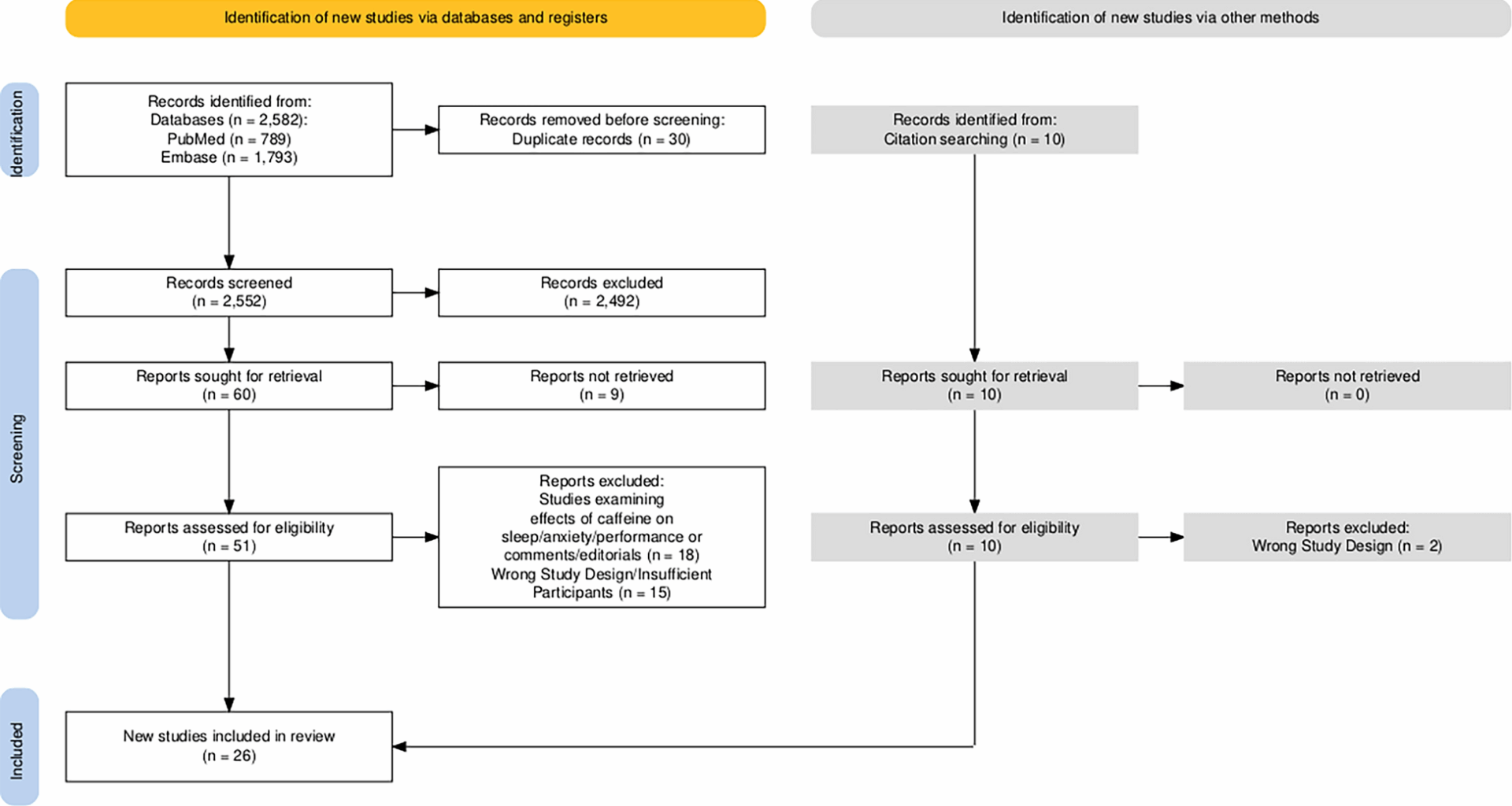
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022. https://doi.org/10.1002/cl2.1230.
Corchero J, Pimprale S, Kimura S, Gonzalez FJ. Organization of the CYP1A cluster on human chromosome 15: implications for gene regulation. Pharmacogenetics. 2001;11(1):1–6. https://doi.org/10.1097/00008571-200102000-00001.
Amin N, Byrne E, Johnson J, et al. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol Psychiatry. 2012;17(11):1116–29. https://doi.org/10.1038/mp.2011.101.
Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9(5):625–37.
Rasmussen BB, Brix TH, Kyvik KO, Brøsen K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12(6):473–8. https://doi.org/10.1097/00008571-200208000-00008.
Sulem P, Gudbjartsson DF, Geller F, Prokopenko I, Feenstra B, Aben KK, Franke B, den Heijer M, Kovacs P, Stumvoll M, Mägi R. Sequence variants at CYP1A1–CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet. 2011;20(10):2071–7.
Kukal S, Thakran S, Kanojia N, Yadav S, Mishra MK, Guin D, Singh P, Kukreti R. Genic-intergenic polymorphisms of CYP1A genes and their clinical impact. Gene. 2023;857:147171.
Cornelis MC. Recent consumption of a caffeine-containing beverage and serum biomarkers of cardiometabolic function in the UK Biobank. Br J Nutr. 2021;126(4):582–90.
Rodenburg EM, Eijgelsheim M, Geleijnse JM, et al. CYP1A2 and coffee intake and the modifying effect of sex, age, and smoking123. Am J Clin Nutr. 2012;96(1):182–7. https://doi.org/10.3945/ajcn.111.027102.
Josse AR, Da Costa LA, Campos H, El-Sohemy A. Associations between polymorphisms in the AHR and CYP1A1-CYP1A2 gene regions and habitual caffeine consumption. Am J Clin Nutr. 2012;96(3):665–71. https://doi.org/10.3945/ajcn.112.038794.
Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011. https://doi.org/10.1371/journal.pgen.1002033.
Genome-wide association studies of coffee intake in UK/US participants of European ancestry uncover cohort-specific genetic associations. Neuropsychopharmacology. https://www.nature.com/articles/s41386-024-01870-x#MOESM2. Accessed 4 Sept 2024.
McMahon G, Taylor AE, Davey Smith G, Munafò MR. Phenotype refinement strengthens the association of AHR and CYP1A1 genotype with caffeine consumption. PLoS ONE. 2014;9(7): e103448. https://doi.org/10.1371/journal.pone.0103448.
Ding R, Shi J, Pabon K, Scotto KW. Xanthines down-regulate the drug transporter ABCG2 and reverse multidrug resistance. Mol Pharmacol. 2012;81(3):328–37. https://doi.org/10.1124/mol.111.075556.
Ross DD, Karp JE, Chen TT, Doyle LA. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood. 2000;96(1):365–8.
Rogers PJ, Hohoff C, Heatherley SV, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35(9):1973–83. https://doi.org/10.1038/npp.2010.71.
Zhong VW, Kuang A, Danning RD, et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet. 2019;28(14):2449–57. https://doi.org/10.1093/hmg/ddz061.
Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86(1):240–4. https://doi.org/10.1093/ajcn/86.1.240.
Martins GL, Guilherme JPLF, Ferreira LHB, de Souza-Junior TP, Lancha AH. Caffeine and exercise performance: possible directions for definitive findings. Front Sports Act Living. 2020. https://doi.org/10.3389/fspor.2020.574854.
Aklillu E, Djordjevic N, Carrillo JA, Makonnen E, Bertilsson L, Ingelman-Sundberg M. High CYP2A6 enzyme activity as measured by a caffeine test and unique distribution of CYP2A6 variant alleles in Ethiopian population. OMICS J Integr Biol. 2014;18(7):446. https://doi.org/10.1089/omi.2013.0140.
Cornelis MC, Munafo MR. Mendelian randomization studies of coffee and caffeine consumption. Nutrients. 2018;10(10):1343. https://doi.org/10.3390/nu10101343.
Pirastu N, Kooyman M, Robino A, et al. Non-additive genome-wide association scan reveals a new gene associated with habitual coffee consumption. Sci Rep. 2016;6:31590. https://doi.org/10.1038/srep31590.
Jin T, Youn J, Kim AN, et al. Interactions of habitual coffee consumption by genetic polymorphisms with the risk of prediabetes and type 2 diabetes combined. Nutrients. 2020;12(8):2228. https://doi.org/10.3390/nu12082228.
Nakagawa-Senda H, Hachiya T, Shimizu A, et al. A genome-wide association study in the Japanese population identifies the 12q24 locus for habitual coffee consumption: The J-MICC Study. Sci Rep. 2018;8(1):1493. https://doi.org/10.1038/s41598-018-19914-w.
Jia H, Nogawa S, Kawafune K, et al. GWAS of habitual coffee consumption reveals a sex difference in the genetic effect of the 12q24 locus in the Japanese population. BMC Genet. 2019;20(1):61. https://doi.org/10.1186/s12863-019-0763-7.
Cao J, Hudziak JJ, Li D. Multi-cultural association of the serotonin transporter gene (SLC6A4) with substance use disorder. Neuropsychopharmacology. 2013;38(9):1737–47. https://doi.org/10.1038/npp.2013.73.
Arnold MR, Williams PH, McArthur JA, et al. Effects of chronic caffeine exposure during adolescence and subsequent acute caffeine challenge during adulthood on rat brain serotonergic systems. Neuropharmacology. 2019;148:257–71. https://doi.org/10.1016/j.neuropharm.2018.12.019.
Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35(2):495–519. https://doi.org/10.1016/j.psc.2012.03.010.
Carlier N, Marshe VS, Cmorejova J, Davis C, Müller DJ. Genetic similarities between compulsive overeating and addiction phenotypes: a case for “food addiction”? Curr Psychiatry Rep. 2015;17:1–1.
Heber D, Carpenter CL. Addictive genes and the relationship to obesity and inflammation. Mol Neurobiol. 2011. https://doi.org/10.1007/s12035-011-8180-6.
Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addict Behav. 2013;38(7):2306–12. https://doi.org/10.1016/j.addbeh.2013.02.012.
Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14(1):60–70. https://doi.org/10.1038/sj.mp.4002086.
Parsian A, Cloninger CR, Zhang ZH. Functional variant in the DRD2 receptor promoter region and subtypes of alcoholism. Am J Med Genet. 2000. https://doi.org/10.1002/1096-8628(20000612)96:3%3c407::aid-ajmg32%3e3.0.co;2-1.
Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6(4):577–82. https://doi.org/10.1093/hmg/6.4.577.
Hirvonen M, Laakso A, Någren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9(12):1060–1.
van Dyck CH, Malison RT, Jacobsen LK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med Off Publ Soc Nucl Med. 2005;46(5):745–51.
Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–54.
Williams LM, Gatt JM, Grieve SM, et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage. 2010;53(3):918–25. https://doi.org/10.1016/j.neuroimage.2010.01.084.
Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. https://doi.org/10.1086/425589.
Clarke TK, Adams MJ, Davies G, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112,117). Mol Psychiatry. 2017;22(10):1376–84. https://doi.org/10.1038/mp.2017.153.
Matoba N, Akiyama M, Ishigaki K, et al. GWAS of 165,084 Japanese individuals identified nine loci associated with dietary habits. Nat Hum Behav. 2020;4(3):308–16. https://doi.org/10.1038/s41562-019-0805-1.
Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. https://doi.org/10.1038/nature14177.
Akiyama M, Okada Y, Kanai M, et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 2017;49(10):1458–67. https://doi.org/10.1038/ng.3951.
Muskiewicz DE, Uhl GR, Hall FS. The role of cell adhesion molecule genes regulating neuroplasticity in addiction. Neural Plast. 2018;2018: e9803764. https://doi.org/10.1155/2018/9803764.
Ishiguro H, Liu QR, Gong JP, et al. NrCAM in addiction vulnerability: positional cloning, drug-regulation, haplotype-specific expression, and altered drug reward in knockout mice. Neuropsychopharmacology. 2006;31(3):572–84. https://doi.org/10.1038/sj.npp.1300855.
Uhl GR, Liu QR, Naiman D. Substance abuse vulnerability loci: converging genome scanning data. Trends Genet. 2002;18(8):420–5. https://doi.org/10.1016/S0168-9525(02)02719-1.
Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet. 1998;81(3):216–21. https://doi.org/10.1002/(SICI)1096-8628(19980508)81:3%3c216::AID-AJMG2%3e3.0.CO;2-U.
Pirastu N, Kooyman M, Traglia M, et al. Association analysis of bitter receptor genes in five isolated populations identifies a significant correlation between TAS2R43 variants and coffee liking. PLoS ONE. 2014;9(3): e92065. https://doi.org/10.1371/journal.pone.0092065.
Kuhn C, Bufe B, Winnig M, et al. Bitter taste receptors for saccharin and acesulfame K. J Neurosci Off J Soc Neurosci. 2004;24(45):10260–5. https://doi.org/10.1523/JNEUROSCI.1225-04.2004.
Ledda M, Kutalik Z, Souza Destito MC, et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum Mol Genet. 2014;23(1):259–67. https://doi.org/10.1093/hmg/ddt404.
Cheng B, Pan C, Cheng S, et al. Whole exome sequencing study identifies novel rare risk variants for habitual coffee consumption involved in olfactory receptor and hyperphagia. Nutrients. 2022;14(20):4330. https://doi.org/10.3390/nu14204330.
Liu J, Wang X, Ma R, et al. AMPK signaling mediates synphilin-1-induced hyperphagia and obesity in Drosophila. J Cell Sci. 2021;134(3): jcs247742. https://doi.org/10.1242/jcs.247742.
Shishido T, Nagano Y, Araki M, et al. Synphilin-1 has neuroprotective effects on MPP+-induced Parkinson’s disease model cells by inhibiting ROS production and apoptosis. Neurosci Lett. 2019;690:145–50. https://doi.org/10.1016/j.neulet.2018.10.020.
Saimaiti A, Zhou DD, Li J, et al. Dietary sources, health benefits, and risks of caffeine. Crit Rev Food Sci Nutr. 2023;63(29):9648–66. https://doi.org/10.1080/10408398.2022.2074362.
Carrillo JA, Benítez J. Caffeine metabolism in a healthy Spanish population: N-acetylator phenotype and oxidation pathways. Clin Pharmacol Ther. 1994;55(3):293–304. https://doi.org/10.1038/clpt.1994.30.
Kot M, Daniel WA. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem Pharmacol. 2008;75(7):1538–49. https://doi.org/10.1016/j.bcp.2007.12.017.
Fulton JL, Dinas PC, Carrillo AE, Edsall JR, Ryan EJ, Ryan EJ. Impact of genetic variability on physiological responses to caffeine in humans: a systematic review. Nutrients. 2018;10(10):1373. https://doi.org/10.3390/nu10101373.
Conger SA, Tuthill LM, Millard-Stafford ML. Does caffeine increase fat metabolism? A systematic review and meta-analysis. Int J Sport Nutr Exercise Metabol. 2022. https://doi.org/10.1123/ijsnem.2022-0131.
Harpaz E, Tamir S, Weinstein A, Weinstein Y. The effect of caffeine on energy balance. J Basic Clin Physiol Pharmacol. 2017;28(1):1–10. https://doi.org/10.1515/jbcpp-2016-0090.
LeBlanc J, Jobin M, Cote J, Samson P, Labrie A. Enhanced metabolic response to caffeine in exercise-trained human subjects. J Appl Physiol. 1985;59(3):832–7. https://doi.org/10.1152/jappl.1985.59.3.832.
Gkouskou KG, Georgiopoulos G, Vlastos I, et al. CYP1A2 polymorphisms modify the association of habitual coffee consumption with appetite, macronutrient intake, and body mass index: results from an observational cohort and a cross-over randomized study. Int J Obes. 2022;46(1):162–8. https://doi.org/10.1038/s41366-021-00972-6.
Sun D, Lu J, Zhang Y, et al. Characterization of a novel CYP1A2 knockout rat model constructed by CRISPR/Cas9. Drug Metab Dispos. 2021;49(8):638–47. https://doi.org/10.1124/dmd.121.000403.
Thompson SM, Rakoczy RJ, Duffy MA, Kiss AJ, McMurray MS. Differential consumption of alcohol, caffeine, and caffeinated alcohol by adolescent rats, and effects on post-adolescent gene expression signatures in the nucleus accumbens and orbitofrontal cortex. Drug Alcohol Depend. 2023;251:110921. https://doi.org/10.1016/j.drugalcdep.2023.110921.
Stonehouse AH, Adachi M, Walcott EC, Jones FS. Caffeine regulates neuronal expression of the dopamine 2 receptor gene. Mol Pharmacol. 2003;64(6):1463–73. https://doi.org/10.1124/mol.64.6.1463.
Horvath G, Adam G, Tuboly G, et al. Caffeine–treat or trigger? Disparate behavioral and long-term dopaminergic changes in control and schizophrenia-like Wisket rats. Physiol Behav. 2021;236:113410. https://doi.org/10.1016/j.physbeh.2021.113410.
Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22(15):6321–4.
dos Santos Sanna PL, Bernardes Carvalho L, dos Santos Afonso CC, et al. Adora2A downregulation promotes caffeine neuroprotective effect against LPS-induced neuroinflammation in the hippocampus. Brain Res. 2024;1833:148866. https://doi.org/10.1016/j.brainres.2024.148866.
Yee M, Maal-Bared G, Ting-A-Kee R, Chwalek M, Mackay-Clackett I, Bergamini M, Grieder TE, van der Kooy D. Segregation of caffeine reward and aversion in the rat nucleus accumbens shell versus core. Eur J Neurosci. 2020. https://doi.org/10.1111/ejn.14718.
Lüscher C, Janak PH. Consolidating the circuit model for addiction. Ann Rev Neurosci. 2021. https://doi.org/10.1146/annurev-neuro-092920-123905.
Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. https://doi.org/10.1016/j.neuropharm.2015.05.009.
Wang J, Dewi L, Peng Y, Hou CW, Song Y, Condello G. Does ergogenic effect of caffeine supplementation depend on CYP1A2 genotypes? A systematic review with meta-analysis. J Sport Health Sci. 2023. https://doi.org/10.1016/j.jshs.2023.12.005.
Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295(10):1135–41. https://doi.org/10.1001/jama.295.10.1135.
Mahdavi S, Palatini P, El-Sohemy A. CYP1A2 genetic variation, coffee intake, and kidney dysfunction. JAMA Netw Open. 2023;6(1): e2247868. https://doi.org/10.1001/jamanetworkopen.2022.47868.
Palatini P, Ceolotto G, Ragazzo F, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27(8):1594. https://doi.org/10.1097/HJH.0b013e32832ba850.
Hermann R, Rostami-Hodjegan A, Zhao P, Ragueneau-Majlessi I. Seeing what is behind the smokescreen: a systematic review of methodological aspects of smoking interaction studies over the last three decades and implications for future clinical trials. Clin Transl Sci. 2023;16(5):742–58. https://doi.org/10.1111/cts.13494.
Hussein NA, Muskiewicz DE, Terrero D, Malla S, Hall FS, Tiwari AK. The effects of drugs of abuse on ABC transporters. In: Patel VB, Preedy VR, editors. Handbook of substance misuse and addictions: from biology to public health. Berlin: Springer International Publishing; 2022. p. 609–34. https://doi.org/10.1007/978-3-030-92392-1_184.
Yang J, Reilly BG, Davis TP, Ronaldson PT. Modulation of opioid transport at the blood-brain barrier by altered ATP-binding cassette (ABC) transporter expression and activity. Pharmaceutics. 2018;10(4):192. https://doi.org/10.3390/pharmaceutics10040192.
Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010;163(6):919–24. https://doi.org/10.1530/EJE-10-0764.
Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genom. 2008;18(7):569. https://doi.org/10.1097/FPC.0b013e32830054ac.
Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(s1):S3–15. https://doi.org/10.3233/JAD-2010-1379.
Aguiar AS, Speck AE, Canas PM, Cunha RA. Neuronal adenosine A2A receptors signal ergogenic effects of caffeine. Sci Rep. 2020;10(1):13414. https://doi.org/10.1038/s41598-020-69660-1.
Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105(4):1067–79. https://doi.org/10.1111/j.1471-4159.2007.05196.x.
Tanner JA, Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J Pers Med. 2017;7(4):18. https://doi.org/10.3390/jpm7040018.
Cornelis MC, van Dam RM. Genetic determinants of liking and intake of coffee and other bitter foods and beverages. Sci Rep. 2021;11(1):23845. https://doi.org/10.1038/s41598-021-03153-7.
Buchwald J, Chenoweth MJ, Palviainen T, et al. Genome-wide association meta-analysis of nicotine metabolism and cigarette consumption measures in smokers of European descent. Mol Psychiatry. 2021;26(6):2212–23. https://doi.org/10.1038/s41380-020-0702-z.
Homan P, Grob S, Milos G, et al. The role of BDNF, leptin, and catecholamines in reward learning in bulimia nervosa. Int J Neuropsychopharmacol. 2015;18(5): pyu092. https://doi.org/10.1093/ijnp/pyu092.
Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56. https://doi.org/10.1016/j.tins.2011.11.004.
Leão RM, Cruz FC, Carneiro-de-Oliveira PE, et al. Enhanced nicotine-seeking behavior following pre-exposure to repeated cocaine is accompanied by changes in BDNF in the nucleus accumbens of rats. Pharmacol Biochem Behav. 2013;104:169–76. https://doi.org/10.1016/j.pbb.2013.01.007.
Corominas-Roso M, Roncero C, Daigre C, et al. Changes in brain-derived neurotrophic factor (BDNF) during abstinence could be associated with relapse in cocaine-dependent patients. Psychiatry Res. 2015;225(3):309–14. https://doi.org/10.1016/j.psychres.2014.12.019.
Green CR, Corsi-Travali S, Neumeister A. The role of BDNF-TrkB signaling in the pathogenesis of PTSD. J Depress Anxiety. 2013. https://doi.org/10.4172/2167-1044.S4-006.
Chandio ZA, Sidiqua A, Khaskheli MI, Waghani A, Metlo WA. Review effect of caffeine overdose. RADS J Biol Res Appl Sci. 2020;11(2):154–8. https://doi.org/10.37962/jbas.v11i2.266.
Fernstrom MH, Bazil CW, Fernstrom JD. Caffeine injection raises brain tryptophan level, but does not stimulate the rate of serotonin synthesis in rat brain. Life Sci. 1984. https://doi.org/10.1016/0024-3205(84)90094-8.
Courtiol E, Menezes EC, Teixeira CM. Serotonergic regulation of the dopaminergic system: implications for reward-related functions. Neurosci Biobehav Rev. 2021;128:282–93. https://doi.org/10.1016/j.neubiorev.2021.06.022.
Clayman CL, Connaughton VP. Neurochemical and behavioral consequences of ethanol and/or caffeine exposure: effects in zebrafish and rodents. Curr Neuropharmacol. 2022;20(3):560–78. https://doi.org/10.2174/1570159X19666211111142027.
Bogdan R, Hatoum AS, Johnson EC, Agrawal A. The genetically informed neurobiology of addiction (GINA) model. Nat Rev Neurosci. 2023;24(1):40. https://doi.org/10.1038/s41583-022-00656-8.
Solinas M, Belujon P, Fernagut PO, Jaber M, Thiriet N. Dopamine and addiction: what have we learned from 40 years of research. J Neural Transm. 2019;126(4):481–516. https://doi.org/10.1007/s00702-018-1957-2.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–12. https://doi.org/10.1038/379606a0.
Kooner JS, Chambers JC, Aguilar-Salinas CA, et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet. 2008;40(2):149–51. https://doi.org/10.1038/ng.2007.61.
Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res. 2013;3(3):114–30. https://doi.org/10.1089/jcr.2013.0016.
Sweeney MM, Weaver DC, Vincent KB, Arria AM, Griffiths RR. Prevalence and correlates of caffeine use disorder symptoms among a United States sample. J Caffeine Adenosine Res. 2020;10(1):4–11. https://doi.org/10.1089/caff.2019.0020.
Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis JAD. 2010;20(Suppl 1):S239-248. https://doi.org/10.3233/JAD-2010-1378.
Moore R, Casale FP, Jan Bonder M, et al. A linear mixed-model approach to study multivariate gene-environment interactions. Nat Genet. 2019;51(1):180–6. https://doi.org/10.1038/s41588-018-0271-0.
Sun N, Wang Y, Chu J, Han Q, Shen Y. Bayesian approaches in exploring gene-environment and gene-gene interactions: a comprehensive review. Cancer Genom Proteom. 2023;20(6 Suppl):669–78. https://doi.org/10.21873/cgp.20414.
Booth N, Saxton J, Rodda SN. Estimates of caffeine use disorder, caffeine withdrawal, harm and help-seeking in New Zealand: a cross-sectional survey. Addict Behav. 2020;109:106470. https://doi.org/10.1016/j.addbeh.2020.106470.
Diagnostic and statistical manual of mental disorders. DSM Library. https://doi.org/10.1176/appi.books.9780890425596.
Amer SA, AlAmri FA, AlRadini FA, et al. Caffeine addiction and determinants of caffeine consumption among health care providers: a descriptive national study. Eur Rev Med Pharmacol Sci. 2023;27(8):3230–42. https://doi.org/10.26355/eurrev_202304_32093.
Verster JC, Koenig J. Caffeine intake and its sources: a review of national representative studies. Crit Rev Food Sci Nutr. 2018. https://doi.org/10.1080/10408398.2016.1247252.
Ennis D. The effects of caffeine on health: the benefits outweigh the risks. 2014.
Treur JL, Taylor AE, Ware JJ, et al. Associations between smoking and caffeine consumption in two European cohorts. Addiction. 2016;111(6):1059–68. https://doi.org/10.1111/add.13298.
Lau-Barraco C, Milletich RJ, Linden AN. Caffeinated alcohol consumption profiles and associations with use severity and outcome expectancies. Addict Behav. 2014;39(1):308–15. https://doi.org/10.1016/j.addbeh.2013.10.017.
Kim MJ, Jin HS, Eom YB. Coffee consumption affects kidney function based on GCKR polymorphism in a Korean population. Nutr Res. 2024;122:92–100. https://doi.org/10.1016/j.nutres.2023.12.008.
Kusic DM, Zajic SC, Gharani N, et al. Genome-wide association study of caffeine consumption using coriell personalized medicine collaborative data. Genet Mol Med. 2023. https://doi.org/10.33425/2689-1077.1020.
Kang J, Jia T, Jiao Z, et al. Increased brain volume from higher cereal and lower coffee intake: shared genetic determinants and impacts on cognition and metabolism. Cereb Cortex. 2022;32(22):5163–74. https://doi.org/10.1093/cercor/bhac005.
Zhou A, Taylor AE, Karhunen V, et al. Habitual coffee consumption and cognitive function: a Mendelian randomization meta-analysis in up to 415,530 participants. Sci Rep. 2018;8(1):7526. https://doi.org/10.1038/s41598-018-25919-2.
Denden S, Bouden B, Haj Khelil A, Ben Chibani J, Hamdaoui MH. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: evidence from a meta-analysis. Genet Mol Res. 2016. https://doi.org/10.4238/gmr.15027487.