Trillions of gut microbes may hold the key to better rest. A new review reveals how shifts in bile acids, neurotransmitters, and gut flora intersect with sleep disorders, providing new insights for dietary and microbiome-based therapies.
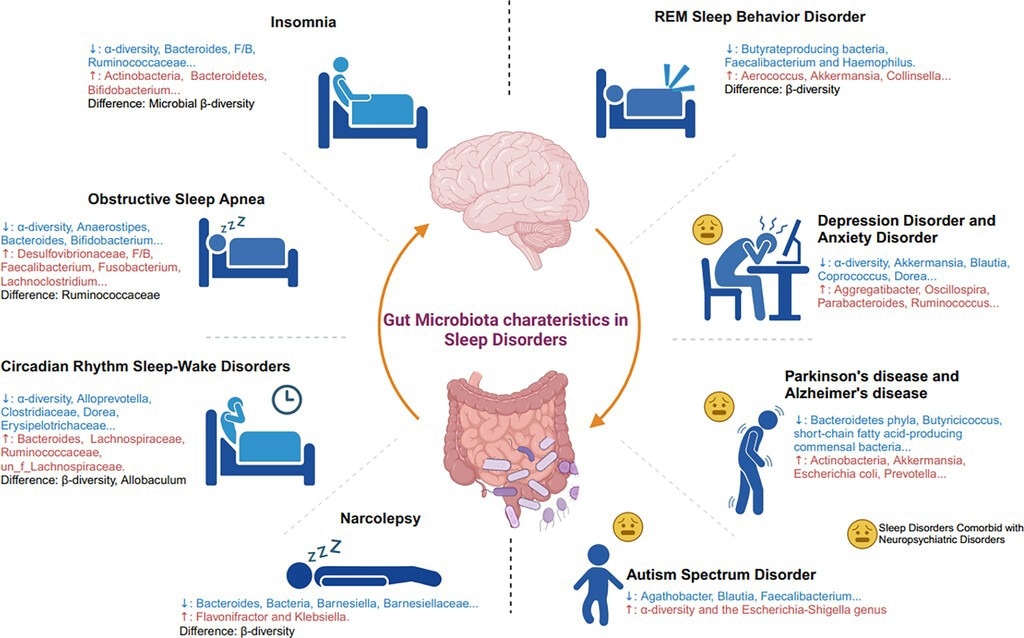
Gut microbiota characteristics in sleep disorders. The figure shows representative characteristics of gut microbiota changes in patients with sleep disorders and those with sleep disorders combined with psychiatric disorders. F/B: the ratio of Firmicutes/Bacteroidetes.
In a recent review published in the journal Brain Medicine, a group of authors synthesized evidence on microbiota-gut-brain interactions related to sleep disorders, aiming to clarify the mechanisms, biomarkers, and microbiome-targeted interventions.
Background
One in three adults reports poor sleep, yet the story may start in the gut. The gastrointestinal (GI) tract harbors trillions of microbes that communicate with the brain through chemical messengers, nerves, and immune signals. Disruptions in the “microbiota-gut-brain axis” are associated with insomnia, obstructive sleep apnea (OSA), and circadian rhythm misalignment, as well as neuropsychiatric conditions where sleep is compromised. Altered microbial diversity and metabolites appear repeatedly, suggesting a shared biology across disorders and real-world factors, including diet and probiotics. Patients, families, and clinicians care because the same pathways shape mood, metabolism, and long-term brain health. Further research is needed to establish causality and optimize targeted, testable therapies.
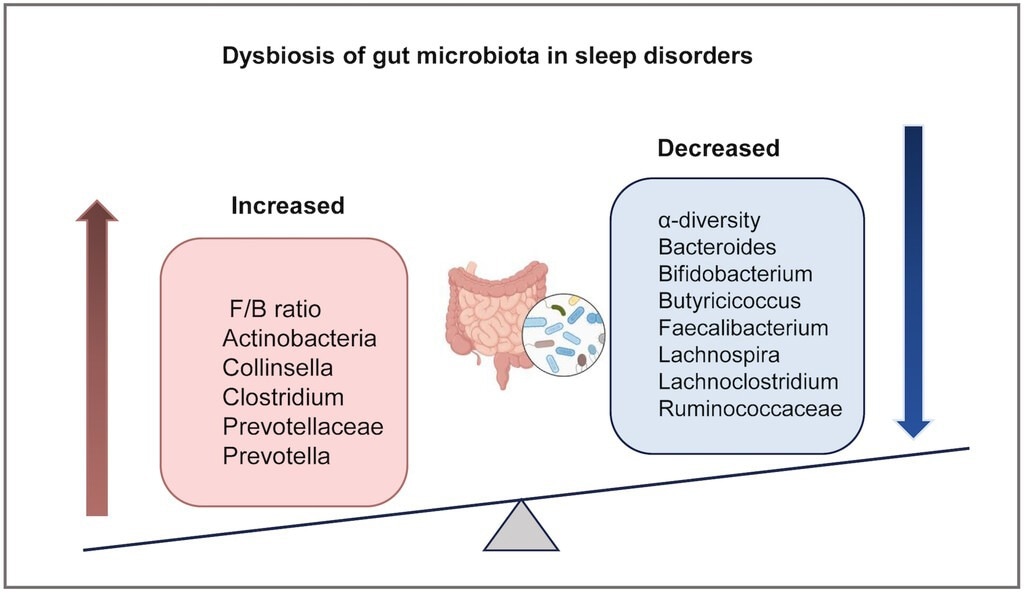
Convergent alterations in gut microbiota across multiple sleep disorders. This schematic summarizes the direction of changes in key bacterial taxa that have been consistently reported in at least two major sleep disorders. F/B: the ratio of Firmicutes/Bacteroidetes.
Evidence Across Sleep Disorders, in Real-Life Terms
Insomnia
People with chronic insomnia often exhibit lower within-sample microbial diversity (alpha diversity) and a shifted community structure (beta diversity), as well as altered bile-acid profiles, including higher primary bile acids (e.g., muricholic and cholic acids) and lower secondary bile acids (e.g., isolithocholic, lithocholic, and ursodeoxycholic). These patterns are linked to taxa such as Ruminococcaceae and cardiometabolic risk, tying a bad night’s sleep to long-term heart and metabolic health, which families plan around. However, specific taxa findings remain heterogeneous across studies, while bile-acid signatures appear more consistent. The paper further emphasizes that these bile acid results were validated in an independent cohort, thereby strengthening their reproducibility.
OSA
In adults and children, OSA is associated with altered alpha and beta diversity, and a reduction in Ruminococcaceae has been repeatedly reported. Faecalibacterium findings are mixed, and several short-chain fatty acid (SCFA)-producing taxa appear to be reduced in severe disease. These gut shifts correlate with the apnea-hypopnea index (AHI) and OSA severity, suggesting a potential biomarker value that requires validation rather than direct monitoring by clinicians.
Circadian rhythm sleep-wake disorders
Shift work and chronic jet lag exhibit compositional changes, including increases in Actinobacteria/Firmicutes and Dorea longicatena, which align with enhanced intestinal permeability and inflammation. This explains the “inflamed” mornings associated with gastrointestinal symptoms and metabolic strain. Human night-shift studies remain small and preliminary, while animal models show more consistent effects on microbial composition and inflammation.
Narcolepsy and rapid eye movement (REM) sleep behavior disorder (RBD)
Narcolepsy type 1 is characterized by a higher abundance of Klebsiella and a lower abundance of beneficial genera, such as Blautia and Lactococcus. Idiopathic RBD exhibits fewer butyrate-producing bacteria, such as Faecalibacterium and Butyricicoccus, and these microbial shifts may precede RBD onset and track disease progression, which is important because RBD can precede Parkinson’s disease (PD).
Neuropsychiatric comorbidity
In autism spectrum disorder (ASD) with sleep problems, diversity indices rise while Faecalibacterium falls, with metabolites shifting toward lower melatonin and higher serotonin, linking daily behavior, sleep, and gut chemistry caregivers recognize.
Mechanisms that Connect the Plate, the Pillow, and the Brain
Bile acids (BAs)
Bile acids (BAs) are cholesterol-derived molecules that help digest fat and also signal to metabolism and immunity. In chronic insomnia, patterns shift toward higher primary and lower secondary BAs, consistent with a microbiota–bile-acid axis that can raise cardiometabolic risk and make poor sleep a whole-body issue.
SCFAs
SCFAs such as acetate, propionate, and butyrate are made when gut microbes ferment dietary fiber. SCFAs influence sleep-relevant physiology; animal studies and early human data suggest a benefit, but the effects are likely to vary by host context and require larger, standardized trials.
Gamma-aminobutyric acid (GABA)
Several gut bacteria, including Lactobacillus and Bifidobacterium, carry glutamate decarboxylase and produce gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter. Antibiotics lower cecal GABA, and oral GABA alters electroencephalography (EEG) responses, suggesting microbe-to-brain calming signals that matter for sleep onset and continuity.
Serotonin (5-hydroxytryptamine [5-HT]) and tryptophan
Over 90% of serotonin (5-HT) is made in the gut. Levels peak during wake and fall in rapid eye movement (REM) sleep. Microbiota steer tryptophan toward 5-HT and influence melatonin synthesis, explaining why late meals or stress can derail sleep.
Hormonal and neural relays
The hypothalamic-pituitary-adrenal (HPA) axis, enteric nervous system (ENS), and vagus nerve connect gut signals to sleep centers. Reduced heart-rate variability (HRV) is common in insomnia and OSA. Vagal pathways are implicated; vagus nerve stimulation (VNS) is under investigation for sleep disorders, and HRV findings align with impaired vagal tone in insomnia and OSA. These circuits likely relay gut signals to sleep–wake centers such as the nucleus tractus solitarius and paraventricular thalamic nucleus.
Interventions and Translational Implications
Prebiotic fiber can reshape bile-acid pools and steady circadian rhythms, useful for shift workers choosing meals. Probiotics and fecal microbiota transplantation (FMT) improved Pittsburgh Sleep Quality Index (PSQI), Insomnia Severity Index (ISI), and Epworth Sleepiness Scale (ESS) scores in chronic insomnia; children with ASD showed lower Sleep Disturbance Scale for Children (SDSC) scores after FMT. Though not first-line, microbiome modulation warrants trials and selective use. In OSA, microbial features track with AHI; in RBD, Butyricicoccus may flag PD. These findings remain preliminary, and larger standardized trials are needed to confirm efficacy. Prioritize equity by ensuring affordable fiber sources, culturally familiar fermented foods, and practical timing for meals, light, and activity.
What Matters for Patients and Families
For patients and families, start with everyday levers like fiber-rich meals, regular daylight exposure, and consistent sleep windows to support SCFAs, melatonin, and healthy vagal tone. Pair airway treatments for OSA with weight management, dietary changes, and microbiome support so symptoms and cardiometabolic risks are addressed together. Dietary and lifestyle measures are supportive, not substitutes for standard medical therapies. Stay alert to early flags, especially in RBD or during shift-work fatigue; GI changes and mood swings may reflect shared gut-brain biology and should be raised in clinic visits for timely evaluation and tailored guidance.
Conclusions
This review shows that sleep disorders consistently align with shifts in gut microbial diversity and metabolites, notably BAs and SCFAs, alongside neurotransmitter-linked pathways involving GABA, serotonin, and melatonin. Mechanistic relays span the HPA axis, ENS, and vagal signaling, matching clinical patterns in insomnia, OSA, circadian disruption, narcolepsy, and RBD. Emerging interventions, from prebiotics to FMT, improve patient-reported sleep outcomes, but larger causal studies and standardized protocols are needed to guide equitable, practical care.
Journal reference:
Wang, Z., Wu, T., Li, J., Lu, T., Yu, Y., Guan, Z., Yuan, G., Lv, Z., Shan, Y., Yan, W., Liu, X., Vitiello, M. V., Yin, Q., Sun, J., & Lu, L. (2025). Brain-gut-microbiota interactions in sleep disorders. Brain Medicine, 1–22. DOI: 10.61373/bm025i.0128,
https://genomicpress.kglmeridian.com/view/journals/brainmed/aop/article-10.61373-bm025i.0128/article-10.61373-bm025i.0128.xml
